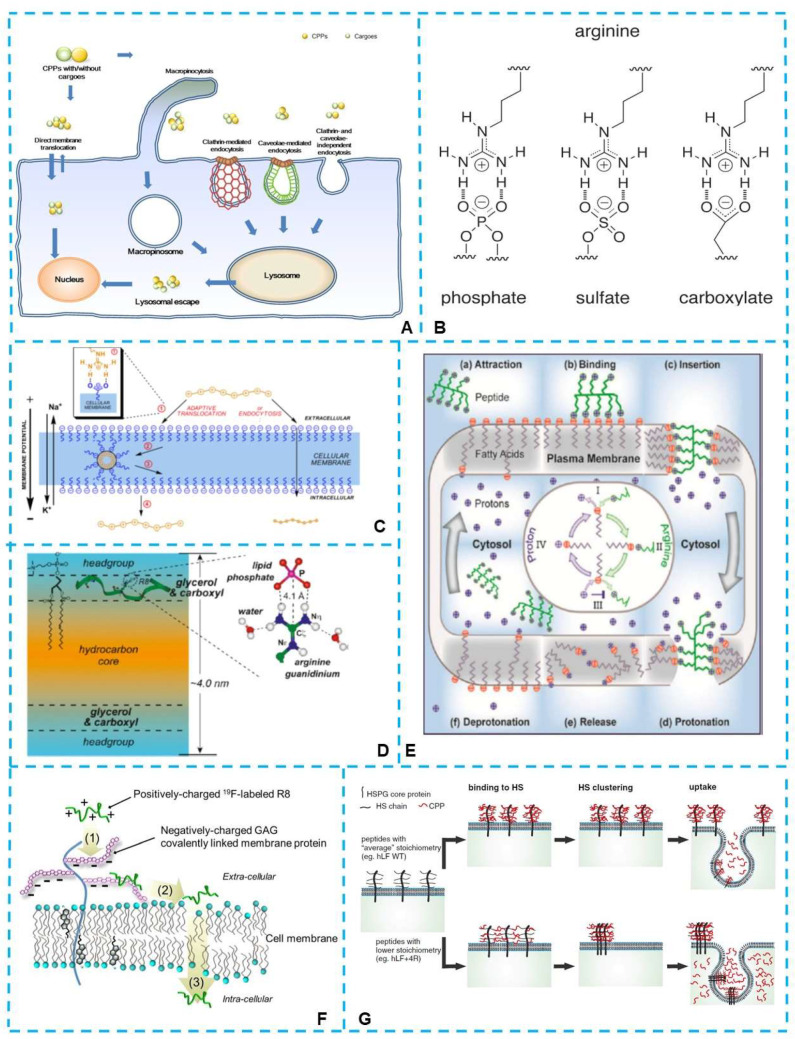
Figure 1.
(A) Different cellular entry routes for either cell-penetrating peptides (CPPs) alone or CPP/cargo complexes. Direct membrane translocation and endocytosis were two major routes which had been proposed [33]. (B) Possible divalent hydrogen bond formation by the side-chain guanidino moiety of arginine with phosphate, sulfate, and carboxylate groups [37]. (C) Mechanisms of the arginine-rich CPPs uptake. The guanidinium group formed a bidentate bond with negative phosphates, sulfates, and carboxylates on the cell surface [38]. (D) Mechanism of the phosphates-related membrane penetration of arginine-rich CPPs, HIV Tat (48–60) [39]. (E) The proposed cellular uptake mechanism for arginine-rich CPPs revealed that deprotonated fatty acids were involved in the membrane penetration process [40]. (F) The mechanism for non-endocytic, energy-independent translocation of 19F-R8 into cells. The mechanism involved (1) binding of 19F-R8 to GAG at the cell surface, followed by (2) the transfer to the cell membrane and (3) the entry into the cytosol [41]. (G) Schematic of the proposed stoichiometry-dependence of uptake. The guanidine groups in arginine formed bidentate hydrogen bonds with negatively charged heparan sulfates. The cross-linking of HS was the driving force for the uptake of arginine-rich CPPs [42].