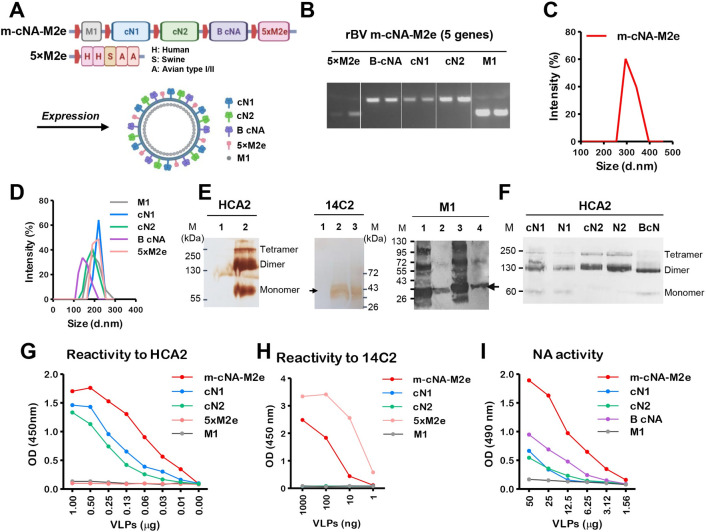
Fig 1. Design and characterization of m-cNA-M2e VLPs containing consensus multi-subtype cNA and tandem repeat 5×M2e.
(A) Scheme diagram of multi-component m-cNA-M2e VLP vaccine expressing M1, multi subtype cNA (cN1, cN2, and B-cNA), and 5×M2e genes under each polyhedrin promoter (red boxes). (B) PCR analysis for confirmation of five genes cloned into the rBV transfer plasmid pFastBac using gene specific primers. (C and D) Size distribution of (C) m-cNA-M2e VLP, (D) mono cN1 VLP, cN2 VLP, and 5×M2e VLP. d.nm: diameter in nanometer. (E) Western blot analysis of m-cNA-M2e and controls using HCA2 (left) mAb specific for pan NA222-230, 14C2 mAb specific for M2e (middle), and M1 (right) specific mAb (ab22396). M: size marker; kDa: kilodalton. VLPs in each line were loaded with 20–30 μg. For HCA2 and 14C2; lane 1 M1 VLP, lane 2 m-cNA-M2e VLP, lane 3 5×M2e VLP. For M1; lane 1 m-cNA-M2e VLP, lane 2 M1 VLP, lane 3 inactivated A/PR8 virus, lane 4 5×M2e VLP. (F) Western blot analysis of monomeric VLP samples. VLP and NA protein samples were loaded with 30 μg and 5 μg respectively. cN1: consensus cN1 VLP, N1: NA protein from A/California/04/2009 H1N1 (BEI, NR-19234), cN2: consensus cN2 VLP, N2: NA protein from A/Brisbane/10/2007 H3N2 (BEI, NR-43784), B-cN: influenza B consensus NA VLP. (G and H) The reactivity of m-cNA-M2e VLP, mono cNA VLPs and 5×M2e VLP to HCA2 (G) or 14C2 (H) mAbs by ELISA. (I) Functional NA activity of m-cNA-M2e VLP and mono cNA VLPs by ELLA.