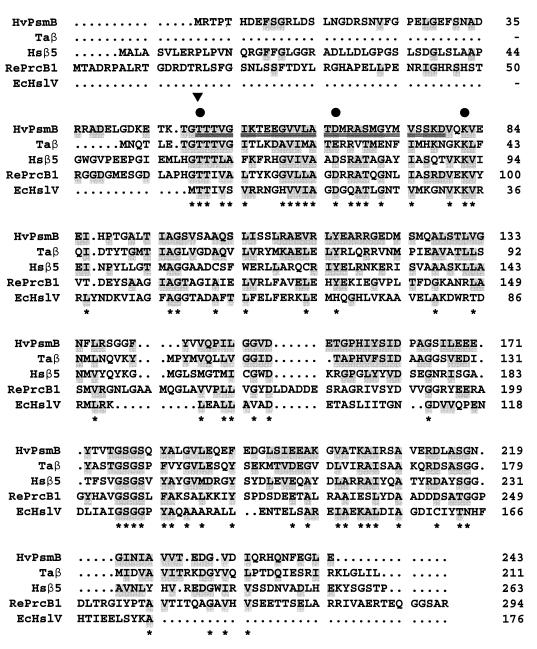
FIG. 7.
Multiple-amino-acid alignment of the PsmB protein (β subunit) of H. volcanii with the β-type proteasome and HslV proteins. Double-underlined amino acid residues are identical to the N-terminal protein sequence of the purified β subunit of a 20S proteasome from H. volcanii. The cleavage site used during maturation of the β-type protein is indicated by an arrowhead above the protein sequence alignment. Conserved residues proposed to be involved in peptide bond hydrolysis are indicated by a filled-in circle above the protein sequence alignment. Abbreviations: Hv, H. volcanii; Ta, T. acidophilum; Hs, Homo sapiens; Re, R. erythropolis; Ec, E. coli. Taβ, Hsβ5 (ɛ, X, MB1), RePrcB1, and EcHslV(ClpQ) sequences were accessed through GenBank protein loci numbers 130867, 4506201, 847769, and 417158, respectively. Residues identical or functionally similar to HvPsmB are shaded. Highly conserved residues are indicated with an asterisk below the protein sequence alignment.