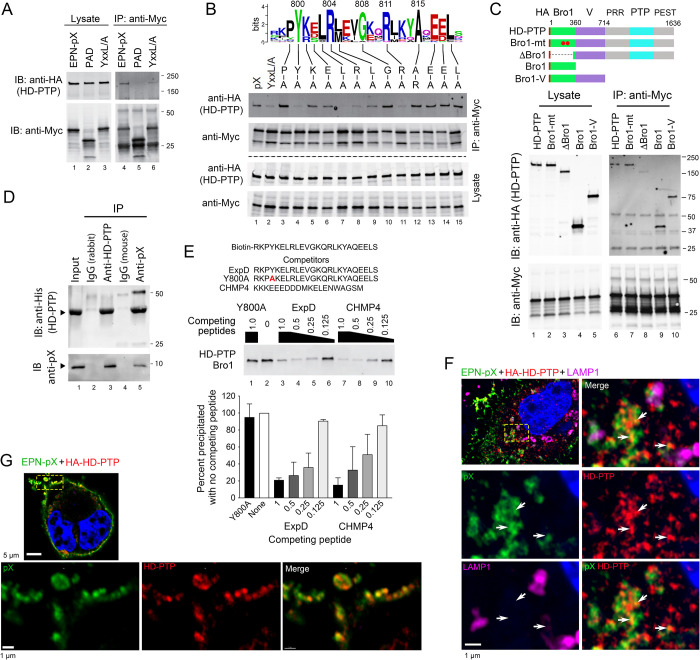
Fig 5. pX interacts directly with the Bro1 domain of the ALIX paralog, HD-PTP.
(A) Co-immunoprecipitation of HA-tagged HD-PTP with EPN-pX. Proteins in lysates of cells expressing EPN-pX, EPN-PAD, or EPN-YxxL/A (Fig 2A) were precipitated with anti-Myc antibody and immunoblotted with antibody to HD-PTP. (B) Co-immunoprecipitation assays of HD-PTP with EPN-pX mutants containing single amino acid substituitions of conserved residues in the ExpD sequence. See Fig 3C. (C) Co-immunoprecipitation assays of EPN-pX with HD-PTP, HD-PTP with L202D and I206D substitutions in the Bro1 domain that eliminate CHMP4 binding (Bro1-mt) [43], and various HD-PTP domain fragments. PRR, proline-rich region; PTP, protein tyrosine phosphatase; PEST, “rich in proline, glutamate, serine and threonine” [51] (D) Co-immunoprecipitation of bacterially-expressed pX and recombinant HD-PTP Bro1 protein (residues 1–360) produced in E. coli. (E) Biotin-tagged ExpD peptide pulldown assay of HD-PTP Bro1 domain (residues 1–360) produced by in vitro transcription/translation in rabbit reticulocyte lysate. See legend to Fig 4G for details. (F) Merged low magnification (top left) and merged and single-channel enlarged super-resolution Airyscan fluorescence images of the area demarcated by the yellow lines showing pX (green), HD-PTP (HA, red), and LAMP1 (magenta) in a 293T cell expressing EPN-pX and HA-HD-PTP. Arrows indicate sites of pX colocalization with HD-PTP. (G) Similar low magnification (top image) and enlarged super-resolution images showing co-localization of pX and HD-PTP along the plasma membrane.