Abstract
Contrast-enhanced MR (CE-MR) imaging is required to improve lesion detection and characterization and to increase diagnostic confidence. This study aims to evaluate the safety, effectiveness, and usage patterns of recently introduced ClariscanTM (gadoterate meglumine) and other macrocyclic gadolinium-based contrast agents (GBCAs) used for magnetic resonance imaging (MRI) of the central nervous system (CNS). Data was obtained from a European multicenter, prospective, observational postmarketing study that included pediatric and adult patients undergoing contrast-enhanced MRI with a GBCA used in routine clinical practice. Safety data was collected by spontaneous patient adverse event (AE) reporting. Effectiveness was assessed via changes in radiological diagnosis, diagnostic confidence, and image quality. 766 patients with CNS-related indications were included from 8 centers across 5 European countries between December 2018 and November 2019. Clariscan (gadoterate meglumine) was used in 66% (503) of exams, Dotarem® (gadoterate meglumine) in 20% (160), Gadovist® (gadobutrol) in 13% (97), and ProHance® (gadoteridol) in 1%. GBCA use increased the diagnostic confidence in 95% (724/766) of patients and a change in radiological diagnosis in 65% (501/766) of patients. The Clariscan-specific data revealed an increase in diagnostic confidence in 94% (472/503) of patients and resulted in a change in radiological diagnosis in 58% (293/503) of patients. Image quality was considered excellent or good in 95% of patients across all GBCAs and in 94% of patients who received Clariscan. No AEs were reported in this cohort including Clariscan. This data demonstrates the excellent safety and efficacy profile of Clariscan and other GBCAs used in MRI examination of the CNS.
Keywords: adverse events, contrast media, gadoterate (Gd-DOTA) Clariscan, image quality, Magnetic resonance imaging
1. Introduction
Magnetic resonance imaging (MRI) contrast agents were introduced in the late 1980s. Gadolinium-based contrast agents (GBCAs) are now used routinely to enhance the sensitivity and specificity of MRI examinations, and over 450 million doses have been administered worldwide.[1–3]
Currently, 6 GBCA molecules are approved for use by the U.S. Food and Drug Administration (FDA). Based on the structure of the ligand, these can be classified into 2 groups: macrocyclic agents (including gadoterate meglumine [Clariscan/ Dotarem], gadobutrol [Gadovist] and gadoteridol [ProHance]) and linear agents (gadodiamide [Omniscan], gadobenate dimeglumine [MultiHance] and gadoxetate [Eovist]). Clariscan, a recently introduced generic agent, contains the same active pharmacological ingredient (API) as Dotarem in the same concentration (0.1 mmol/kg) and equivalent formulation. The inactive ingredients (preservatives, buffers, etc) in both products are the same. Clariscan has been approved for use in 70 countries with over 7 million patient doses shipped globally.[1,3–7]
The recent concerns from gadolinium retention and the European Medicines Agency (EMA) decision to suspend the marketing authorization of linear GBCAs (with exceptions) has led to a steady decline in the use of linear agents in the USA.[8] Unlike the EMA, the USA FDA maintained the marketing authorization of the linear agents and asked the contrast media manufacturers of both linear and macrocyclic agents to conduct safety assessments of their product(s) through human and animal studies.[9]
The choice of GBCA for a patient in clinical practice is complex and often subject to local variability in availability of resources, local protocols, physician expertise, patient expectations, financial constraints, and physicochemical profiles, including molecule stability and adverse events.[1,3]
To our knowledge, there is no prospective study mapping the use of the 4 macrocyclic GBCAs since the EMA suspended the marketing authorization of linear agents in Europe in 2017 and the introduction of a first generic GBCA in the USA.[7,8] With this background, it is important to understand the usage patterns of GBCAs in clinical practice, including referral details, indications, dosing, diagnostic confidence, and their safety.
The objective of this multi-national, prospective, observational study was to evaluate the safety, effectiveness, and usage patterns of recently introduced Clariscan™ (gadoterate meglumine) and other macrocyclic gadolinium-based contrast agents (GBCAs) used for magnetic resonance imaging (MRI) of the central nervous system (CNS).
2. Materials and Methods
2.1. Study design
The data for this study has been obtained from a larger scale European study, a cross-sectional multicenter observational study with prospective recruitment performed in patients scheduled for gadolinium contrast-enhanced magnetic resonance imaging (CE-MRI) as part of their routine clinical workup.[10] The study was registered on https://clinicaltrials.gov with identifier NCT01523873.
Eight centers across 5 European countries (France, Germany, Italy, Poland, and Spain) participated, and a total of 2118 consenting patients underwent CE-MRI examinations between December 2018 and November 2019. Centers participating in the current study required: (1) an independent decision to include Clariscan in the formulary for MR examinations and (2) the ability to maintain electronic patient data records to enable cumulative data reporting at end of the study. A minimum of 3 months recruitment period and 50 patients per site were targeted to warrant adequate representation.
The present study is a subanalysis of the data from the above European study, to assess the real-world safety and efficacy of GBCAs, including Clariscan for CNS (brain and spine) examinations at recommended/on label doses in the USA.[10]
2.2. Patient selection
Enrolled patients were adult and pediatric patients who were scheduled to undergo routine MRI with intravenous (IV) administration of contrast agents for CNS-related indications (brain, spine) at recommended/on label doses in US. Eligible patients received information about the study and a written consent and IRB approval was obtained.
2.3. Gadolinium-based contrast agents
Gadolinium-based contrast agents utilized by the participating centers for MRI exams of the CNS included gadoterate meglumine (Clariscan™; GE Healthcare), gadoteridol (ProHance®; Bracco Imaging), gadobutrol (Gadovist®; Bayer Healthcare) and gadoterate meglumine (Dotarem®; Guerbet). In each participating center, IV administration of GBCA was performed according to local standard protocols.
2.4. Clinical data retrieval
Patient demographics, working diagnoses, relevant medical histories, medications, referral details, indications for MRI examination, and details regarding administration of contrast agents were recorded on a standardized data collection form for each individual patient by trained study staff.
2.5. MRI effectiveness assessments
The assessment of effectiveness included changes in radiological diagnosis (yes/no), diagnostic confidence ratings, and MR image quality. The local radiologist assessed whether the contrast-enhanced MR (CEMR) images changed the radiological diagnosis in each patient. Diagnostic confidence was assessed by the local radiologist on a 0–100 percent scale both for the nonenhanced images (confidence before CEMR) and again after contrast-enhanced images (confidence after CEMR) were obtained. Image quality was reported on a 4-point scale (poor, fair, good, excellent) based on previously described scales for MRI.[11,12]
2.6. Safety monitoring
All included patients who received GBCAs were followed up in each center and AEs were recorded with details regarding diagnosis, onset date, severity (mild, moderate, or severe) and outcome. Spontaneously reported patient adverse events (AEs) were documented, and classified in terms of severity, course of treatment and latency (immediate: <1h postinjection, delayed: 1h−7d postinjection). The local radiologist assessed the likelihood that an AE was related to GBCA administration as follows: not related, related (doubtfully or possibly), or not assessable. AEs doubtfully or possibly related to GBCA administration were defined as adverse drug reactions (ADRs). AEs were summarized using the current MedDRA coding system. The data were recorded by the local investigators in all 8 eligible centers.
2.7. Statistical analysis
Statistical analysis of the data was performed using SAS Software Version 9.4 (SASVR Institute Inc., Cary, NC). Quantitative (continuous) data was reported as means and standard deviations (SD) or medians and ranges. Qualitative (binary) data was reported as raw numbers, frequencies, and 95% confidence intervals. Descriptive analysis was complemented by explorative statistical tests (ANOVA for continuous endpoints, Chi-Square tests for categorical endpoints).
3. Results
3.1. Study cohort
A total of 2188 patients were enrolled in the original European study between December 2018 and November 2019. Of these 2188 patients, 902 patients met the CNS-related indications. Patients with doses over 0.10 mmol/kg were excluded (off label for USA) from this study. Thus, the subset contains 766 patients undergoing CNS imaging at the general/US recommended doses (up to 0.10 mmol/Kg) (Table 1). The majority of patients in this subset were adults; 19–59 years of age [446 patients (58%)] and 314 patients ≥ 60 years (41 %) and female (57%). The mean body mass index (BMI) was 26 kg/m2 (range 15.6–58.6), with the following BMI categories, underweight and normal 322 (42%), overweight 330 (43%), obese and morbidly obese 114 (15 %) (Table 1). There were no significant differences in BMI among the different GBCAs used (P value = 0.3).
Table 1.
Demographic data and baseline characteristics of study population.
| Subjects recruited, n (%) | 766 | (100) |
|---|---|---|
| Country, n (%) | ||
| Poland | 338 | (44) |
| Italy | 83 | (11) |
| Germany | 167 | (22) |
| Spain | 58 | (7.6) |
| France | 120 | (16) |
| Sex, n (%) | ||
| Female | 434 | (57) |
| Male | 332 | (43) |
| Age (y, mean (SD)) | 53.1 | (16) |
| Age category, n (%) | ||
| 0–18 | 6 | (0.8) |
| 19–59 | 446 | (58) |
| ≥60 | 314 | (41) |
| Height (m, mean (SD)) | 1.7 | (0.1) |
| Weight (kg, mean (SD)) | 75.7 | (16) |
| BMI (kg/m2, mean (SD)) | 26.4 | |
| Underweight (BMI < 18.5) | 4 | (0.5) |
| Normal (BMI ≥ 18.5 to < 25) | 318 | (42) |
| Overweight (BMI ≥ 25 to < 30) | 330 | (43) |
| Obese (BMI ≥ 30 to < 40) | 105 | (14) |
| Morbidly obese (BMI ≥ 40) | 9 | (1.2) |
| Comorbidity, n (%) | ||
| Hypertension | 166 | (22) |
| Malignancy/cancer | 64 | (8.4) |
| Allergy | 64 | (8.4) |
| Diabetes mellitus | 47 | (6.1) |
| Neurologic symptom | 53 | (6.9) |
| Autoimmune disease | 25 | (1.2) |
| Renal impairment | 11 | (1.4) |
| Hepatic impairment | 2 | (0.3) |
| Other | 33 | (4.3) |
| Premedication, n (%) | ||
| Steroids | 5 | (0.6) |
| Antihistamines | 3 | (0.4) |
| Concomitant medications, n (%) | ||
| Antihypertensives | 150 | (20) |
| Chemotherapy | 49 | (6.4) |
| Antidiabetic drugs | 41 | (5.4) |
| Other reported | 101 | (13) |
| No co-medication | 443 | (58) |
About half of the patients in this cohort (373 (49%)) had documented comorbidities. The most common was hypertension (22%), followed by history of malignancy and allergic conditions (8.4% each), and neurologic symptoms (6.9%). Concomitant medications were reported in 35% of patients, mostly antihypertensive (20%), chemotherapy (6.4%) and antidiabetic medications (5.4%) (Table 1).
There were 6 patients with a reported prior allergic-like reaction to contrast agents (5 patients to iodinated contrast agents and 1 patient to GBCA). As premedication, steroids were administered in 0.6%[5] and antihistamines in 0.4%[3] of the cases. Renal impairment was reported in 11 cases (1.4%), while hepatic impairment was seen in 2 patients (0.3%).
3.2. Pattern and quality of referral
The cohort consisted of 4.4% (34 cases) emergency procedures, 62% (476 cases) referred for routine diagnosis, and 33% (256 cases) follow-up procedures for a known disease. Referral information was considered well detailed with a clear medical question in 104 cases (14%), satisfactory in 641 cases (84%) and insufficient in 21 cases (2.7%). In terms of satisfactory level of medical information, there was no statistically significant difference among these examined cohorts. (P value = 0.5).
3.3. GBCA and MRI exam details
The GBCAs dose was ≤ 0.10 mmol/kg with median GBCA volume of 14 ml, (range 3–25 ml). Gadoterate meglumine was used in most of the patients, with Clariscan being used in 503 patients (66%) and Dotarem in 160 patients (21%), followed by gadobutrol (Gadovist) in 97 patients (12%) and gadoteridol (Prohance) in 6 patients (1%) (Table 2).
Table 2.
Injections details by GBCA*
| N (%) | |
|---|---|
| N | 766 |
| Type of exam | |
| Routine | 476 (62%) |
| Follow-up | 256 (33%) |
| Emergency | 34 (4.4%) |
| 1.5T (vs 3T) | 634 (83%) |
| MR angiography | 53 (6.9%) |
| GBCA volume (ml median) (min-max) | 14 ml (3–25) |
| GBCA dose (mmol/kg) | 0.10≤ |
| GBCA administered | |
| Clariscan | 503 (66%) |
| Dotarem | 160 (21%) |
| Gadovist | 97 (13%) |
| ProHance | 6 (0.8%) |
1.5 Tesla MRI was used in 83 % (n = 634) of the studies included in our analysis and 17% (n = 132) were performed using 3 Tesla MRI. Majority of Clariscan (n = 415 (83%)), Dotarem (n = 132 (83%)), Gadovist (n = 87 (90%)) studies were performed using 1.5 Tesla MRI. There was no statistically significant difference among GBCAs group (P = .2) (Table 2).
3.4. GBCA effectiveness
The use of GBCAs increased the confidence in diagnosis in 95% of the examinations. Mean confidence in diagnosis increased from 51 to 90% and median confidence from 50 to 93% (0–100 scale) and resulted in a change in the radiological diagnosis in 65% of the patients (Table 3), (Fig. 1).
Table 3.
Changes in radiological diagnosis and confidence after CE-MRI/MRA* and image quality.
| Clariscan | Dotarem | Gadavist | ProHance | |
|---|---|---|---|---|
| N | 503 | 160 | 97 | 6 |
| Changes in radiological diagnosis, n (%) | 293 (58%) | 126 (79%) | 79 (81%) | 3 (50%) |
| Increased confidence in diagnosis, n (%) | 472 (94%) | 153 (96%) | 94 (97%) | 5 (83%) |
| Confidence before CEMR (median) | 52 | 41 | 39 | 51 |
| Confidence after CEMR (median) | 95 | 91 | 91 | 100 |
| Image quality | ||||
| Poor | 1 (0.2%) | 0 | 0 | 0 |
| Fair | 31 (6.2%) | 2 (1.3%) | 5 (5.2%) | 0 |
| Good | 178 (35%) | 70 (44%) | 51 (53%) | 4 (67%) |
| Excellent | 293 (58%) | 88 (55%) | 41 (42%) | 2 (33%) |
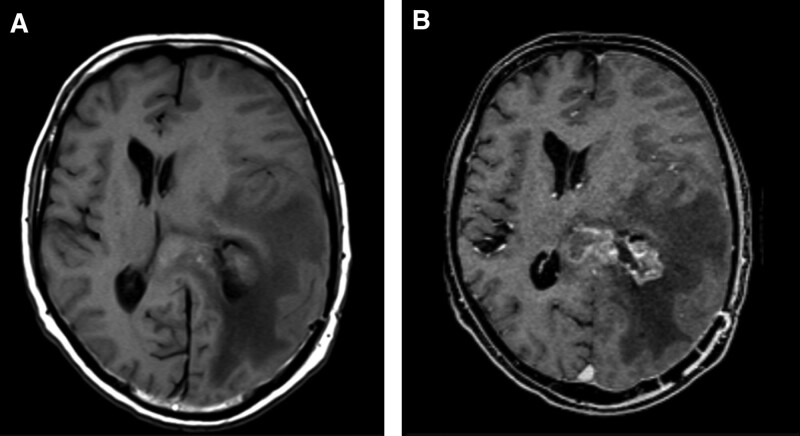
Figure 1.
High grade brain glioma pre (a) and post administration of contrast (b), in a 32-year-old male patient. A poorly defined Heterogeneous left parietal mass in T1 without contrast, associated with marked vasogenic edema and midline shift to the contralateral side. After administration of contrast (Clariscan, 0.10 mmol/kg), it shows heterogeneous enhancement and allows to distinctly define the border of the left periventricular parietal mass with ependymal extension with marked vasogenic edema and midline shift. Image quality was reported as good by site investigator.
Overall, image quality was considered excellent or good in 95% of the cases (excellent (n = 424) 55%, good (n = 303) 40%, fair (n = 38) 5%, and poor (n = 1) 0.1 %. Quality of the images with Clariscan was considered excellent or good or in (471) 94% of the cases, excellent 58% (n = 293), good 35% (n = 178), fair 6.2% (n = 31), poor 0.2%.[1] There was no statistically significant difference in the quality of Clariscan images compared to other CE-MR images (P = .70).
3.5. GBCA safety
There were 6 patients with a reported history of prior allergic-like reaction to contrast material. In our subset of MRI examinations, there were no reported AEs post administration of Clariscan or any other GBCAs.
4. Discussion
This multi-center, prospective real-world study involving adult and pediatric patients demonstrated an excellent safety and efficacy profile of the recently introduced gadolinium-based contrast agent (GBCA) gadoterate meglumine Clariscan™ and other GBCAs used for CNS imaging.
In this cohort, the consistent with prior studies (90–99.7%).[6,13–17] Image quality was good to excellent in 95% of the cases in our cohort, which is comparable amongst all GBCAs despite the difference in relaxivity. This real-world study complements the data obtained from a randomized control trial by Maravilla et al (REMIND study) that demonstrated current administration of GBCAs had a significant effect on patient care by improving the diagnostic confidence in more than 95% of cases when comparing nonenhanced to contrast-enhanced MRI. Additionally, GBCA administration in this cohort affected/changed the radiological diagnosis in more than 65% of examinations. Overall, these findings reinforce the clinical benefits of GBCA use with US recommended doses (up to 0.10 mmol/Kg) for patients undergoing MR examinations for CNS indications.
We found an overall image quality (fair, good, and excellent) rate of 99.8% for MR examinations using gadoterate meglumine (Clariscan), the noninferiority of gadoterate meglumine (Dotarem) vs gadobutrol (Gadovist) for overall visualization and characterization of primary brain tumors despite the higher relaxivity and molarity of gadobutrol.[13]
A common strategy for increasing MRI sensitivity in the detection of brain lesions is increasing the dose of the contrast agents.[16,18] However, present data suggest that using a standard dose (<0.1 mmol/kg) is sufficient to accomplish high quality diagnostic imaging in majority of the patients. This result is important, because GBCAs are sometimes administered at more than this standard dose, which could result in safety concerns related to the use of GBCAs, including gadolinium retention and NSF.
Our study found that Clariscan is a well-tolerated GBCA, with no AEs reported in this cohort of patients undergoing CNS examination (brain and spine). However, in the complete European cohort that included higher doses of GBCAs and different indications, the adverse event rate was 0.19% (0.05% serious AEs). These findings corroborate with prior studies of gadoterate meglumine safety.
A study of 3444 patients following gadoterate meglumine (Dotarem) administration by Ishiguchi and Takahashi yielded an overall 1.16% incidence of AEs including 0.12% serious AEs.[1] Other studies of Dotarem by Soyer et al (overall AE incidence 0.12%; serious AEs 0.03% in 35,499 patients) and Maurer et al (overall AE rate of 0.34%; serious AEs < 0.01% in 84,621 patients) yielded similar results.[14,15]
Additional studies have examined AEs following administration of other GBCAs. Power et al reported an incidence of 0.32% allergic-like reactions in 32,991 patients after gadobutrol injection.[19] Morgan et al found an overall adverse reaction rate of 0.67% (0.01% severe) in 28,078 patients following gadoteridol injection.[20] A major European prospective registry with 72,839 GBCA enhanced cardiac MRs reported a total incidence of AEs of 0.36% and severe AEs of 0.03%,[19] based on their data gadoterate had the lowest incidence of AEs (OR 0.89), that was not statistically different from gadobutrol (reference OR 1), while gadoteridol was found to have a statistically significant higher incidence of AEs (OR 3.58).[21] In reflection of these AE incidences, the introduction of Clariscan as a new brand of gadoterate does not seem to be associated with a higher rate of adverse events or a potential Weber effect.[22]
Other known safety issues with GBCAs include gadolinium retention in tissues and nephrogenic systemic fibrosis (NSF). In vitro studies have shown that macrocyclic GBCAs including gadoterate meglumine, gadobutrol, and gadoteridol have higher stability constants and have a lower risk of gadolinium dissociation compared to linear GBCAs including gadodiamide and gadobenate.[23] To our knowledge, very few single-agent nephrogenic systemic fibrosis (NSF) cases have been associated with the macrocyclic agent gadobutrol, while no unconfounded cases of NSF have been reported for gadoterate, gadobenate, or gadoteridol.[24] The RESCUE Study by Deray et al, which assessed the safety of gadoterate-enhanced MRI compared to unenhanced MRI on 514 patients with impaired renal function, including end-stage or dialysis patients, found that gadoterate did not affect renal function and was a safe contrast agent in patients with chronic kidney disease.[5]
Our study included a broad range of patients, with heterogenous patient demographics and medical histories. Notes from referring physicians were found to vary in detail and accuracy, with only 12% of cases with well-detailed information with a clear medical question, demonstrating that there seems to be room for improvement in clinical practice, sufficient information in 84% of cases, and insufficient information in 4.8% of the cases. As previously reported, accurate referral notes are key to ensure that radiologists perform the appropriate exam including GBCA use and parameters.[25] Additionally, paucity of details regarding patient history and medications can negatively impact patient safety when alternative sources of data to the referral notes are not available.[26]
This study had several limitations. First, the cohort represented a limited number of cases, thus the study was underpowered to fully examine the rate of adverse events (AEs) and to compare different macrocyclic agents. AEs may have been underestimated if they developed after the patient left the radiology department. Nevertheless, this is unlikely to impact the reported rate of serious adverse events, which most likely occur as immediate reactions. Second, this study was observational and therefore was not designed to compare GBCAs for differences in efficacy or assess the reliability of changed diagnosis with each disease for different types of contrast agents. Additional prospective larger multicenter studies are needed for direct GBCA comparisons and generalizability of our findings to determine the rate of AEs more accurately.
5. Conclusions
In conclusion, this study of usage patterns of GBCA in a real-world clinical setting indicates that Clariscan and other GBCAs are safe and effective when intravenously administered in adults and children for MR examination of the CNS (brain and spine). These findings corroborate and complement previously published clinical trials evaluating safety and efficacy of GBCAs.
Author contributions
AHB and JM: Data interpretation, first manuscript drafting and manuscript review. Authors read and approved the final manuscript.
Abbreviations:
- AE =
- adverse event
- BMI =
- body mass index
- CE-MR =
- contrast enhanced magnetic resonance
- CNS =
- central nervous system
- GBCA =
- gadolinium-based contrast agent
- MRA =
- magnetic resonance angiography
- MRI =
- magnetic resonance imaging
- NSF =
- nephrogenic systemic fibrosis
- SAE =
- serious adverse event
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding: The study was sponsored by GE Healthcare Ltd and included financial support for the costs of database creation, data management and statistical analysis. Availability of data and materials the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Heshmatzadeh Behzadi A, Mcdonald J. Gadolinium-based contrast agents for imaging of the central nervous system: a multicenter European prospective study. Medicine 2022;101:34(e30163).
The authors have no conflicts of interest to disclose.
AHB and JM have been involved with GE Healthcare as consultants.
References
- [1].Robert JM, Levine D, Weinreb J, et al. Gadolinium retention: a research roadmap from the 2018 NIH/ACR/RSNA workshop on gadolinium chelates. Radiology. 2018;289:517–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Russell EJ, Geremia GK, Johnson CE, et al. Multiple cerebral metastases: detectability with Gd-DTPA-enhanced MR imaging. Radiology. 1987;165:609–17. [DOI] [PubMed] [Google Scholar]
- [3].Wahsner J, Gale EM, Rodríguez-Rodríguez A, et al. Chemistry of MRI contrast agents: current challenges and new frontiers. Chem Rev. 2019;119:957–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fukunaga T, Fujii S, Inoue C, et al. Accuracy of semiquantitative dynamic contrast-enhanced MRI for differentiating type II from type I endometrial carcinoma. J Magn Reson Imaging. 2015;41:1662–8. [DOI] [PubMed] [Google Scholar]
- [5].Deray G, Rouviere O, Bacigalupo L, et al. Safety of meglumine gadoterate (Gd-DOTA)-enhanced MRI compared to unenhanced MRI in patients with chronic kidney disease (RESCUE study). Eur Radiol. 2013;23:1250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chang DH, Pracros JP. Safety of gadoterate meglumine in over 1600 children included in the prospective observational SECURE study. Acta Radiol. 2019;60:1450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Clariscan Summary of Product Characteristics. European Heads of Medicines Agencies. Available at: https://mri.cts-mrp.eu/Human. [access date June 2,2021].
- [8].EMA’s Fnal Opinion Confrms Restrictions on Use of Linear Gadolinium Agents in Body Scans EMA/457616/2017. 2017. Available at: https://www.ema.europa.eu/en/medicines/human/referrals/gadolinium-containingcontrast-agents. [access date 2 June 2021].
- [9].FDA Identifes No Harmful Effects to Date with Brain Retention of Gadolinium-Based Contrast Agents for MRIs. 2017. Available at: https://www.fdagov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-identifes-no-harmful-efects-date-brain-retention-gadolinium. [access date 2 June 2021].
- [10].Jakobsen JA, Quattrocchi CC, Müller FHH, et al. Patterns of use, effectiveness and safety of gadolinium contrast agents: a European prospective cross-sectional multicentre observational study. BMC Med Imaging. 2021;21:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Maravilla KR, San-Juan D, Kim SJ, et al. Comparison of gadoterate meglumine and gadobutrol in the MRI diagnosis of primary brain tumors: a double-blind randomized controlled intraindividual crossover study (the REMIND Study). AJNR Am J Neuroradiol. 2017;38:1681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hansmann J, Michaely HJ, Morelli JN, et al. Enhancement characteristics and impact on image quality of two gadolinium chelates at equimolar doses for time-resolved 3-Tesla MR-angiography of the calf station. PLoS One. 2014;9:e99079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Herborn CU, Honold E, Wolf M, et al. Clinical safety and diagnostic value of the gadolinium chelate gadoterate meglumine (Gd-DOTA). Invest Radiol. 2007;42:58–62. [DOI] [PubMed] [Google Scholar]
- [14].Ishiguchi T, Takahashi S. Safety of gadoterate meglumine (Gd-DOTA) as a contrast agent for magnetic resonance imaging: results of a post-marketing surveillance study in Japan. Drugs R D. 2010;10:133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Soyer P, Dohan A, Patkar D, et al. Observational study on the safety profile of gadoterate meglumine in 35,499 patients: the SECURE study. J Magn Reson Imaging. 2017;45:988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maurer M, Heine O, Wolf M, et al. Tolerability, and diagnostic value of gadoteric acid in the general population and in patients with risk factors: results in more than 84,000 patients. Eur J Radiol. 2012;81:885–90. [DOI] [PubMed] [Google Scholar]
- [17].Oudkerk M, Sijens PE, Van Beek EJ, et al. Safety and efficacy of dotarem (Gd-DOTA) versus magnevist (Gd-DTPA) in magnetic resonance imaging of the central nervous system. Invest Radiol. 1995;30:75–8. [DOI] [PubMed] [Google Scholar]
- [18].Ba-Ssalamah A, Nöbauer-Huhmann I, Pinker K, et al. Effect of contrast dose and field strength in the magnetic resonance detection of brain metastases. Invest Radiol. 2003;38:415–22. [DOI] [PubMed] [Google Scholar]
- [19].Power S, Talbot N, Kucharczyk W, et al. Allergic-like reactions to the MR imaging contrast agent gadobutrol: a prospective study of 32 991 consecutive injections. Radiology. 2016;281:72–7. [DOI] [PubMed] [Google Scholar]
- [20].Morgan DE, Spann JS, Lockhart ME, et al. Assessment of adverse reaction rates during gadoteridol-enhanced MR imaging in 28,078 patients. Radiology. 2011;259:109–16. [DOI] [PubMed] [Google Scholar]
- [21].Uhlig J, Lücke C, Vliegenthart R, et al. Acute adverse events in cardiac MR imaging with gadolinium-based contrast agents: results from the European Society of Cardiovascular Radiology (ESCR) MRCT registry in 72,839 patients. Eur Radiol. 2019;29:3686–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hartnell NR, Wilson JP. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy. 2004;24:743–9. [DOI] [PubMed] [Google Scholar]
- [23].Ide´e JM, Port M, Robic C, et al. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging. 2009;30:1249–58. [DOI] [PubMed] [Google Scholar]
- [24].Kanal E, Maravilla K, Rowley HA. Am J Neuroradiol. 2014;35:2215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Abbas M, Omer A, Hamad M. Adequacy of clinical information on radiology request cards from medical assessment unit, Clinical Audit. Nucl Med Biomed Imaging. 2016;1:5–6. [Google Scholar]
- [26].Obara P, Sevenster M, Travis A, et al. Evaluating the referring physician’s clinical history and indication as a means for communicating chronic conditions that are pertinent at the point of radiologic interpretation. J Digit Imaging. 2015;28:272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]