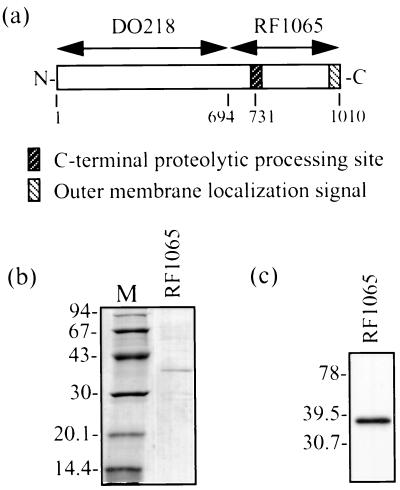
FIG. 1.
(a) Diagram of E. coli BrkA clones used in this study. Numbers below the boxes refer to amino acids. (b) SDS-PAGE and Coomassie blue staining showing pooled fractions of BrkA C-terminal protein obtained by denaturing Ni2+ chromatography after dialysis. Dialysis was performed slowly at 4°C against decreasing concentrations of urea and finally against 0.1% Triton X-100–10 mM Tris (pH 8.0). M, low-molecular-weight markers (Pharmacia) (molecular sizes are in kilodaltons). (c) Western immunoblot of BrkA C-terminal protein (same as in panel b). Detection was performed with an anti-BrkA C-terminal protein monoclonal antibody. Kaleidoscope Prestained Standards (Bio-Rad) were used for molecular size determination (molecular sizes are in kilodaltons).