Abstract
Background
The updated Veterans Aging Cohort Study (VACS) Index 2.0 combines general and human immunodeficiency virus (HIV)–specific biomarkers to generate a continuous score that accurately discriminates risk of mortality in diverse cohorts of persons with HIV (PWH), but a score alone is difficult to interpret. Using data from the North American AIDS Cohort Collaboration (NA-ACCORD), we translate VACS Index 2.0 scores into validated probability estimates of mortality.
Methods
Because complete mortality ascertainment is essential for accurate calibration, we restricted analyses to cohorts with mortality from the National Death Index or equivalent sources. VACS Index 2.0 components were ascertained from October 1999 to April 2018. Mortality was observed up to March 2019. Calibration curves compared predicted (estimated by fitting a gamma model to the score) to observed mortality overall and within subgroups: cohort (VACS/NA-ACCORD subset), sex, age <50 or ≥50 years, race/ethnicity, HIV-1 RNA ≤500 or >500 copies/mL, CD4 count <350 or ≥350 cells/µL, and years 1999–2009 or 2010–2018. Because mortality rates have decreased over time, the final model was limited to 2010–2018.
Results
Among 37230 PWH in VACS and 8061 PWH in the NA-ACCORD subset, median age was 53 and 44 years; 3% and 19% were women; and 48% and 39% were black. Discrimination in NA-ACCORD (C-statistic = 0.842 [95% confidence interval {CI}, .830–.854]) was better than in VACS (C-statistic = 0.813 [95% CI, .809–.817]). Predicted and observed mortality largely overlapped in VACS and the NA-ACCORD subset, overall and within subgroups.
Conclusions
Based on this validation, VACS Index 2.0 can reliably estimate probability of all-cause mortality, at various follow-up times, among PWH in North America.
Keywords: VACS Index 2.0, calibration, mortality, HIV
Based on calibration curves that compared VACS Index 2.0 predicted mortality to observed mortality, overall and within important subgroups, the VACS Index 2.0 can reliably estimate probability of all-cause mortality over time among people with HIV in North America.
Current antiretroviral therapy (ART) is highly active against human immunodeficiency virus (HIV), minimally toxic, and easy for people with HIV (PWH) to take [1, 2]. Adherence to ART allows most PWH to achieve viral suppression and CD4 T-cell immune recovery [3, 4]. Yet, PWH continue to experience poorer health outcomes (specifically non-AIDS events) than uninfected counterparts [5–8], and disparities in life expectancy persist [9]. The observed shift from AIDS morbidity and mortality to non-AIDS conditions prompted the development of the Veterans Aging Cohort Study (VACS) Index as a tool that incorporates general health (age, hemoglobin, Fibrosis-4 Index for Liver Fibrosis [FIB-4], estimated glomerular filtration rate [eGFR], hepatitis C virus [HCV]), and HIV-specific (HIV-1 RNA and CD4 cell count) clinical data to characterize overall disease burden and reflect risk of mortality [10]. The VACS Index 1.0 was developed in VACS using Veterans Health Administration (VHA) national electronic health record data routinely collected on PWH, and it has been shown to discriminate risk of mortality in various samples of PWH. Since its inception and validation, the VACS Index 1.0 has consistently demonstrated its utility in discriminating outcomes including hospitalization [11], medical intensive care admission [11], cardiovascular disease [12], fragility fractures [13], and cognitive function [14, 15].
The VACS Index 1.0 provides well-calibrated risk estimates to assist with medical and personal decision making and is available online at MDCalc (https://www.mdcalc.com/veterans-aging-cohort-study-vacs-index) [10]. To improve the ability of the VACS Index to discriminate changing mortality risk for an individual, the VACS Index 2.0 added clinical predictors (body mass index [BMI], total white blood cell count, and albumin) and the use of continuous instead of categorical variables. VACS Index 2.0 was developed in VACS and externally validated and shown to provide excellent discrimination among other European and North American cohorts participating in the Antiretroviral Therapy Cohort Collaboration (ART-CC) [16].
While the VACS Index 2.0 discriminates risk of mortality effectively, the raw index score is difficult to interpret for an individual; therefore, calibration is needed for translating an index score to an accurate probability of mortality. Calibration requires nearly complete mortality assessment which is not assured in the United States (US) without the use of the National Death Index (NDI) or an equivalent source.
The North American AIDS Cohort Collaboration (NA-ACCORD) comprises multiple North American cohorts, including VACS [17]. We included those cohorts that use mortality endpoints from the NDI or equivalent sources. In this study, we used data from NA-ACCORD to (1) confirm the superior mortality discrimination of VACS Index 2.0 over VACS Index 1.0 overall and within important patient subgroups; (2) translate raw VACS Index 2.0 scores into individualized estimates of the probability of mortality over time; and (3) validate the accuracy of the individualized estimates overall and within important subgroups.
METHODS
Study Population
NA-ACCORD is a multisite collaboration of interval and clinical cohort studies in the US and Canada and represents the North American region of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) [17]. VACS is a participating cohort within NA-ACCORD and was used to develop the VACS Index 2.0. Therefore, we separated VACS from the other NA-ACCORD cohorts for this analysis to demonstrate the generalizability of findings to patients outside the VHA. VACS and NA-ACCORD have been described in detail [16–18].
In addition to VACS, we included the other NA-ACCORD cohorts that routinely collected the laboratory data required for creating the VACS Index 2.0 and use the NDI to track mortality. We required NDI data because (1) there is documented underascertainment of mortality when only the Social Security Administration dataset is used [19–21]; and (2) mortality prediction depends upon the overall mortality rate in the cohort [22–24]. This further limited inclusion to a subset of 3 US clinical cohorts in NA-ACCORD in addition to VACS. VACS uses mortality data from a combination of sources available in the VHA (Social Security Administration, Center for Medicare and Medicaid Services, VHA inpatient deaths, and the Veterans Affairs Death Beneficiary database) with accuracy comparable to NDI [25, 26]. Mortality was available up to 31 March 2019 for VACS and 30 June 2017 for the NA-ACCORD subset. VACS and NA-ACCORD are approved by affiliated institutional review boards.
VACS Index 2.0 Score
The development and internal validity of the VACS Index 2.0 have been described in detail elsewhere [16]. The VACS Index 2.0 includes age, BMI, and the following routinely monitored laboratory tests: CD4 cell count, HIV-1 RNA, hemoglobin, platelets, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, white blood cell count, albumin, and HCV status. Composite markers of liver and renal injury (FIB-4 and eGFR) were computed. FIB-4, composed of AST, ALT, platelets, and age, has been validated as an indicator of liver fibrosis [27]. eGFR (using the Chronic Kidney Disease Epidemiology Collaboration equation, composed of serum creatinine, age, sex, and race) is a validated indicator of impaired renal function [28]. HCV status was defined as positive if the patient ever had a positive antibody test or detectable virus before the baseline date.
We obtained inpatient and outpatient laboratory values and BMI for each visit date at least 1 year after ART initiation from 1 October 1999 to 26 April 2018 for VACS and 1 January 2000 to 31 December 2015 for the NA-ACCORD subset. We randomly selected a laboratory date (baseline date) for each patient to represent participants with different levels of HIV disease severity. Values obtained prior to the visit date were allowed to carry forward for up to 180 days, resulting in complete information for 75% of visits. In sensitivity analysis, allowing values to carry forward for 1 year, 87% of visits had complete data.
Statistical Analyses
Using combined VACS and the NA-ACCORD subset, VACS Index 1.0 and 2.0 were calculated for each person at their baseline date. Follow-up time ended at the first date of the following: 5 years after cohort entry, last date mortality data were available for each cohort, or date of death if death occurred within 5 years of baseline. To confirm that the VACS Index 2.0 improves mortality risk discrimination over VACS Index 1.0, we compared Harrell’s C-statistics from VACS Index 1.0 and 2.0 Cox proportional hazards models overall and among the following subgroups: cohort (VACS, NA-ACCORD subset), sex (men, women), race/ethnicity (black, Hispanic, white), age (<50 years, ≥50 years), HIV-1 RNA (≤500 copies/mL, >500 copies/mL), CD4 count (<350 cells/µL, ≥350 cells/µL), and calendar year (1999–2009, 2010–2018).The ≤500 vs >500 HIV-1 RNA threshold was used because of variation in detectable cutoffs between sites and over time within the VHA. Lower thresholds were not used until later periods (<50 copies/mL was not available in VHA until around 2005–2006 and HIV-1 RNA ≤500 copies/mL remained the cutoff for several sites into the year 2010). We intended to use a relevant threshold to complete a comprehensive analysis during the entire window given that we did not identify a single dominant threshold. We acknowledge this granularity is lacking in real-world clinical data from a national cohort. Calendar year was included as a subgroup to determine whether the mortality rate changed over time.
To evaluate the calibration of the VACS Index 2.0 among the subgroups listed above, we first predicted 5-year all-cause mortality using a parametric (gamma) survival regression model using continuous VACS Index score 2.0 as the only predictor. Observed mortality was estimated using the Kaplan-Meier method. For each 5-point interval of score (collapsed if necessary, to maintain at least 5 deaths and 10 survivors in each interval), we calculated Kaplan-Meier mortality estimates and 95% confidence intervals (CIs). Next, 5-year mortality predictions were compared graphically with observed mortality for all of the subgroups.
For the final mortality risk estimates, we used the more recent 2010–2018 years to predict 1- through 5-year mortality, again using the gamma survival model predicting all-cause mortality using VACS Index score 2.0 as the only predictor. This model provided the equation used for calculating 1- through 5-year predicted mortality for each value of the VACS Index 2.0 scores.
Five-year mortality hazard ratios and 95% CIs per 5-point VACS Index 2.0 increment were calculated overall and for 1999–2009 and 2010–2018 subgroups. For additional context, we graphed the distribution of the sample by the VACS Index 2.0 for the 2010–2018 timeframe as this shows the proportion of the sample that falls into the higher-risk VACS Index 2.0 groups.
RESULTS
Characteristics of the Population
There were 37230 VACS and 8061 NA-ACCORD subset PWH included in the analytic dataset (Supplementary Materials). The NA-ACCORD subset does not overlap with the ART-CC cohorts included in the initial VACS Index 2.0 validation [16]. Approximately half (52%) of the randomly selected baseline dates were in 2010–2018. Among VACS PWH, there were 9393 deaths and median time on ART was 4.3 years (interquartile range [IQR],2.2–8.1 years). The median age of those in VACS was 53 years (IQR,46–60 years); 97% were male; and 48% were black, 40% white, 8% Hispanic, and 3% other race/ethnicity (Table 1). Among the NA-ACCORD subset PWH, there were 1082 deaths and median time on ART was 3.9 years (IQR,2.1–7.0 years). The median age of those in the NA-ACCORD subset was 44 years (IQR, 37–50 years); 81% were male; and 41% were white, 39% black, 16% Hispanic, and 5% other race/ethnicity. (Table 1) Median observation time was similar between VACS (4.1 years [IQR,1.8–5.0 years]) and the NA-ACCORD subset (4.4 years [IQR,2.3–5.0 years]; P<.001).
Table 1.
Characteristics of the Veterans Aging Cohort Study and North American AIDS Cohort Collaboration Subset
| Characteristic | VACS | NA-ACCORD | P Value | ||
|---|---|---|---|---|---|
| (n=37230) | (n=8061) | ||||
| Year at baseline, median (IQR) | 2011 | (2006–2015) | 2009 | (2005–2012) | <.0001 |
| Year of ART initiation, median (IQR) | 2004 | (1999–2010) | 2004 | (1999–2008) | <.0001 |
| Years on ART, median (IQR) | 4.3 | (2.2–8.1) | 3.9 | (2.1–7.0) | <.0001 |
| Age at visit, y, median (IQR) | 53 | (46–60) | 44 | (37–50) | <.0001 |
| Male sex, No. (%) | 36286 | (97) | 6495 | (81) | <.0001 |
| Race/ethnicity, No. (%) | |||||
| White | 15047 | (40) | 3275 | (41) | <.0001 |
| Black | 17955 | (48) | 3105 | (39) | |
| Hispanic | 3000 | (8) | 1291 | (16) | |
| Other/unknown | 1228 | (3) | 390 | (5) | |
| CD4 count, cells/µL, median (IQR) | 466 | (265–695) | 428 | (248–645) | <.0001 |
| HIV-1 RNA >500 copies/mL, No. (%) | 7953 | (21) | 1992 | (25) | <.0001 |
| Hemoglobin, g/dL, median (IQR) | 14.0 | (12.6–15.1) | 14.0 | (12.5–15.1) | .0668 |
| FIB-4, No. (%) | |||||
| <1.45 | 20225 | (54) | 5865 | (73) | <.0001 |
| 1.45–3.25 | 12641 | (34) | 1643 | (20) | |
| >3.25 | 4364 | (12) | 553 | (7) | |
| eGFR, mL/min, median (IQR) | 88 | (70–103) | 100 | (83–113) | <.0001 |
| HCV infection, No. (%) | 9243 | (25) | 1988 | (25) | .7561 |
| Albumin, g/dL, median (IQR) | 4.0 | (3.6–4.3) | 4.2 | (3.9–4.5) | <.0001 |
| WBC count, K/mL, median (IQR) | 5.6 | (4.4–7.1) | 5.5 | (4.3–6.9) | .0034 |
| BMI, kg/m2, median (IQR) | 25.5 | (22.5–29.0) | 25.3 | (22.5–28.6) | .0142 |
| VACS Index Scorea, median (IQR) | |||||
| 1.0 | 28.0 | (13.0–49.0) | 18.0 | (6.0–36.0) | <.0001 |
| 2.0 | 50.4 | (37.4–66.9) | 45.3 | (34.6–60.1) | <.0001 |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; eGFR, estimated glomerular filtration rate; FIB-4, Fibrosis-4 Index for Liver Fibrosis; HCV, hepatitis C virus; HIV-1, human immunodeficiency virus type 1; IQR, interquartile range; NA-ACCORD, North American AIDS Cohort Collaboration; VACS, Veterans Aging Cohort Study; WBC, white blood cell.
For calculating the VACS Index Scores 1.0 and 2.0, refer to the Supplementary Materials.
Discrimination of VACS Index 2.0 in VACS and the NA-ACCORD Subset
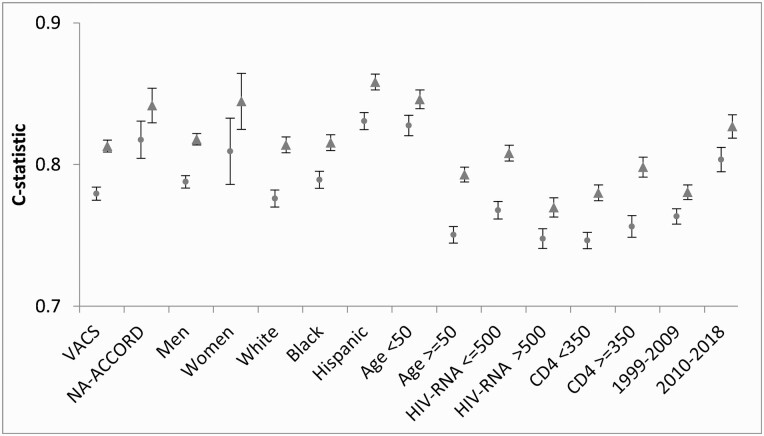
The VACS Index 2.0 demonstrated greater discrimination of all-cause mortality for PWH than VACS Index 1.0. C-statistics were higher for the VACS Index 2.0 compared with VACS Index 1.0 overall (0.819 [95% CI, .815–.823] vs 0.788 [95% CI, .784–.793]); for those in VACS (C-statistic = 0.813 [95% CI, .809–.817] vs 0.779 [95% CI, .775–.784]), NA-ACCORD subset (C-statistic = 0.842 [95% CI, .830–.854] vs 0.818 [95% CI, .804–.831]), and for all other subgroups (Figure 1). Among the subgroups, for VACS Index 2.0 C-statistics were higher for the NA-ACCORD subset vs VACS, for women vs men, for those younger compared to older, for those of Hispanic ethnicity compared to those of black or white race, with HIV-1 RNA ≤500 vs >500 copies/mL, and for calendar years 2010–2018 compared to 1999–2009 (Figure 1).
Figure 1.
Discrimination of 5-year all-cause mortality for Veterans Aging Cohort Study (VACS) Index 1.0 (circles on the left) and VACS Index 2.0 (triangles on the right), in combined VACS and North American AIDS Cohort Collaboration data, by subgroup (n=45291), 1999–2018. Abbreviations: HIV, human immunodeficiency virus; NA-ACCORD, North American AIDS Cohort Collaboration.
Calibration of VACS Index 2.0 in VACS and the NA-ACCORD Subset
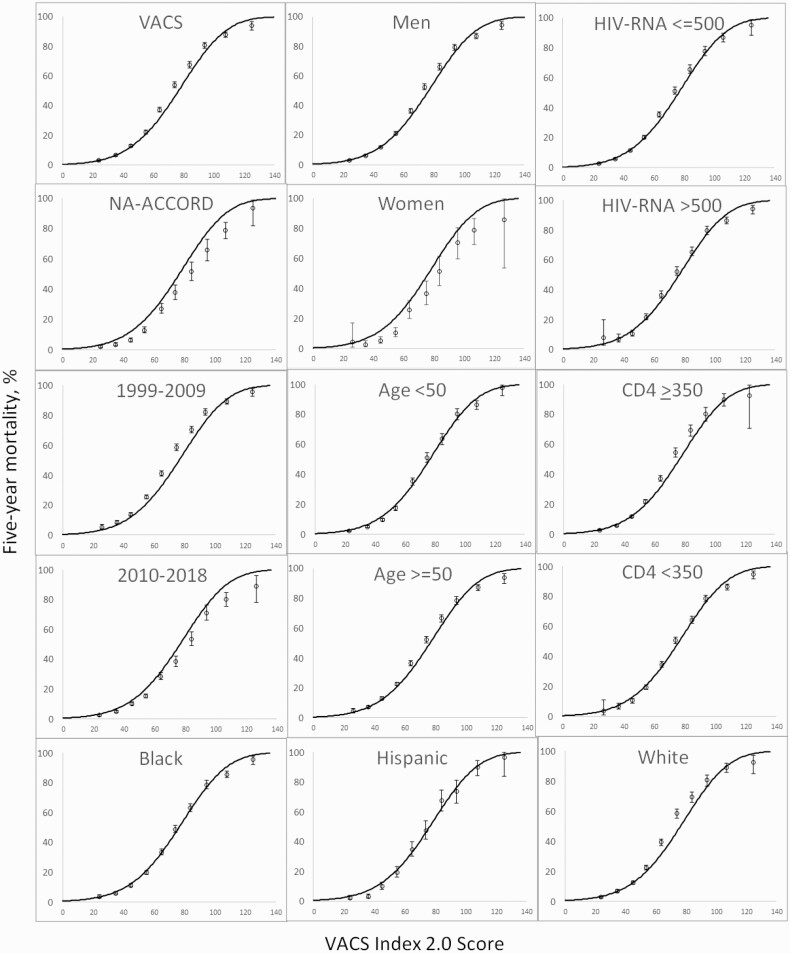
A parametric survival model demonstrated similar 5-year predicted and observed mortality. The overall predicted mortality based upon VACS Index 2.0 was similar to observed mortality among VACS and the NA-ACCORD subset; men and women; those of black, Hispanic, and white race/ethnicity; those aged <50 years and those ≥50 years; those with HIV-1 RNA ≤500 copies/mL and those with >500 copies/mL; and calendar years 1999–2009 and 2010–2018 (Figure 2).
Figure 2.
Five-year all-cause mortality rates by Veterans Aging Cohort Study (VACS) Index 2.0 for important subgroups, 1999–2018. Observed 5-year mortality is shown with 95% confidence intervals. Solid lines reflect predicted mortality calculated using combined VACS and North American AIDS Cohort Collaboration subset (n=45291). Abbreviations: HIV, human immunodeficiency virus; NA-ACCORD, North American AIDS Cohort Collaboration.
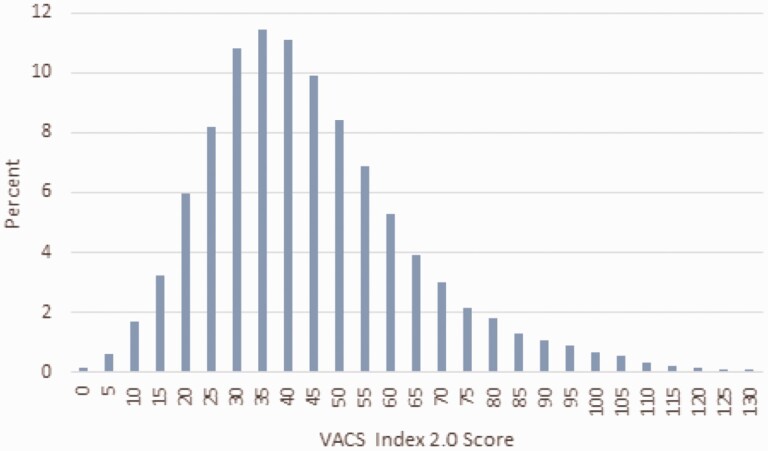
A substantial proportion of the 2010–2018 sample falls into the higher-risk groups with 55% having a VACS Index 2.0 score of >40, and 22% have a VACS Index 2.0 score of >60 (Figure 3). The overall 5-year mortality hazard ratios per 5-point increment of VACS Index 2.0 score are shown in the Supplementary Materials.
Figure 3.
Distribution of combined Veterans Aging Cohort Study (VACS) and North American AIDS Cohort Collaboration subset for 2010–2018 by VACS Index 2.0 (n=23614).
Translating the VACS Index 2.0 Into Mortality Risk Estimates Among People With HIV, 2010–2018
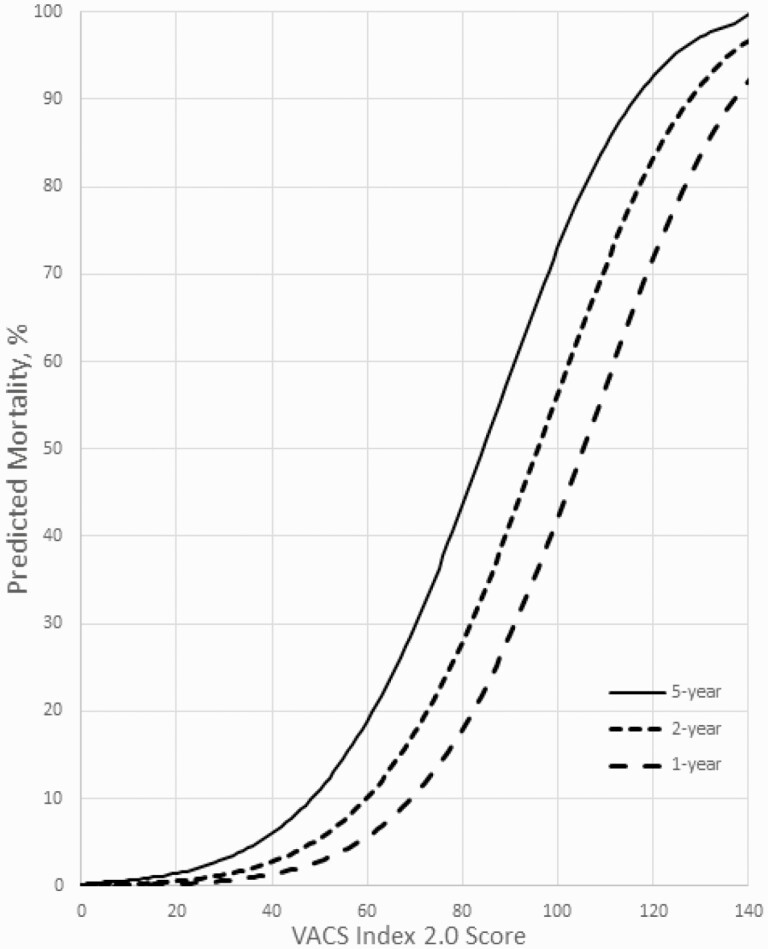
Because mortality rates changed dramatically from 1999 to 2010, we limited the final calibration models to 2010–2018. Gamma models were run to predict 1- through 5-year mortality with VACS Index 2.0 score as the only predictor (Figure 4 and Table 2). For example, for VACS Index 2.0 score of 40, predicted mortality was 1% at 1 year, 3% at 2 years, and 6% at 5 years. For a score of 60, predicted mortality was 6% at 1 year, 10% at 2 years, and 20% at 5 years. For a score of 100, predicted mortality was 42% at 1 year, 56% at 2 years, and 74% at 5 years. Except at the lowest and highest VACS Index 2.0 levels, 5 points on the VACS Index 2.0 translates to an approximately 6.5% increase in predicted 5-year mortality.
Figure 4.
Predicted 1-, 2-, and 5-year all-cause mortality by Veterans Aging Cohort Study (VACS) Index 2.0 for 2010–2018, calculated using combined VACS and North American AIDS Cohort Collaboration (NA-ACCORD) subset (n=23614).
Table 2.
Predicted Mortality for Years 1–5 by Veterans Aging Cohort Study (VACS) Index 2.0 Score Using Combined VACS and North American AIDS Cohort Collaboration Subset Data (n=23614)
| VACS Index 2.0a | 1 Year (%) | 2 Year (%) | 3 Year (%) | 4 Year (%) | 5 Year (%) |
|---|---|---|---|---|---|
| 10 | 0.1 | 0.3 | 0.4 | 0.6 | 0.7 |
| 20 | 0.3 | 0.6 | 0.9 | 1.3 | 1.6 |
| 30 | 0.6 | 1.3 | 2.0 | 2.7 | 3.3 |
| 40 | 1.4 | 2.8 | 4.1 | 5.3 | 6.4 |
| 50 | 2.9 | 5.5 | 7.8 | 9.8 | 11.7 |
| 60 | 5.7 | 10.2 | 13.8 | 17.0 | 19.8 |
| 70 | 10.5 | 17.5 | 22.9 | 27.3 | 31.1 |
| 80 | 18.0 | 28.0 | 35.1 | 40.5 | 45.0 |
| 90 | 28.7 | 41.3 | 49.5 | 55.5 | 60.1 |
| 100 | 42.2 | 56.3 | 64.6 | 70.2 | 74.3 |
Abbreviation: VACS, Veterans Aging Cohort Study.
For calculating the VACS Index Score 2.0, refer to the Supplementary Materials.
DISCUSSION
In this large North American multicohort analysis using validated mortality endpoints, we confirm the superior mortality discrimination of VACS Index 2.0 over 1.0, and translate VACS Index 2.0 scores into estimates of the probability of mortality over time. The ability to accurately identify PWH who may best benefit from intensive multimodal interventions is essential given current resource limitations. The VACS Index is currently used by several healthcare systems to identify and target the sickest patients for team meetings and discussions directly with patients to improve care management [29]. The VACS Index is also useful for identifying when end-of-life planning may be indicated [29].
Compared to VACS Index 1.0, 2.0 discrimination was better for all subgroups, particularly those with undetectable HIV-1 RNA (C-statistic = 0.77 vs 0.81) and those ≥50 years of age (C-statistic = 0.75 vs 0.77). C-statistics for the VACS Index 2.0 meet or surpass those reported for prognostic indices for all-cause mortality commonly used in clinical practice, including validated indices predicting all-cause mortality among aging HIV-uninfected individuals [22, 23]. Of note, for VACS Index 2.0, discrimination was better in more recent years 2010–2018 (0.83) compared to 1999–2009 (0.78).
When we fit the gamma model to the overall data (1999–2018) in VACS, we found that predicted and observed mortality rates were closely aligned over the 5 years of observation—both overall and within important subgroups (Figure 2). Notably, predicted and observed mortality were completely aligned for men, those <50 and ≥50 years of age, and those with and without detectable HIV-1 RNA. However, the model overpredicted probability of mortality for women, perhaps because overall mortality rates are lower for women than men in the US and/or because the VACS Index was developed in a dataset in which women are underrepresented. The model modestly overpredicted mortality probability in the NA-ACCORD subset. Finally, the model also modestly overpredicted mortality in the more recent time interval (2010–2018). Because both discrimination and calibration were better in the 2010–2018 data and because mortality rates have substantially declined in this interval, we fit the final gamma model to the more recent timeframe (Figure 4).
VACS Index 2.0 will be made accessible for clinical and research purposes in the following ways. We will collaborate with MDCalc to post a web-based calculator, as we have already done for VACS Index 1.0 (https://www.mdcalc.com/veterans-aging-cohort-study-vacs-index). The VACS Index 1.0 calculator has been accessed >88000 times. Furthermore, MDCalc is developing a direct implementation in electronic health record–based decision support systems, which would obviate the need to enter data.
There were some limitations to this study. We included only NA-ACCORD cohorts that used the NDI to ascertain mortality, even though these criteria limited the number of cohorts and sample size. This limitation was necessary because of documented underascertainment of mortality when only the Social Security Administration dataset is used [19–21], which would lead to underestimation of mortality. Most of the sample is male, which may limit translation to female cohorts, and the predicted mortality rates seemed to be less well calibrated in women. Similarly, we were unable to evaluate the VACS Index 2.0 in other underrepresented subpopulations such as transgender persons. Although women and those of Hispanic ethnicity made up a minority of the sample, we also consider the inclusion of >2500 women and >4200 of Hispanic ethnicity to be a strength of this study. The HCV component of the VACS Index 2.0 does not incorporate treatment or sustained virologic response information. However, we think that FIB-4 is a better indicator of liver disease than a more granular HCV variable because it reflects varying levels of liver injury associated with HCV in addition to other sources for liver injury such as hepatitis B and fatty liver disease. Additionally, the HCV weight in the VACS Index equation is relatively weak compared with the weight of FIB-4. We included only variables that would be widely available in clinical practice and research databases and could also be accurately and reliably measured. However, we do also believe that societal factors could cause harm through the pathways included in the VACS Index 2.0.
The VACS Index 2.0 estimates are increasingly important as the population of PWH steadily ages and overall physiologic frailty drives morbidity and mortality. The VACS Index 2.0 can help identify PWH who may be able to safely stretch out intervals between clinical encounters or possibly transfer to primary care as well as help identify PWH in whom it may be time to begin end-of-life discussions. The VACS Index 2.0 provides highly discriminating and well-calibrated probability of mortality estimates that are accurate in diverse subgroups and invaluable for medical and personal decision making.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
APPENDIX
NA-ACCORD Collaborating Cohorts and Representatives.
AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson and Ronald J. Bosch.
AIDS Link to the IntraVenous Experience: Gregory D. Kirk.
Emory-Grady HIV Clinical Cohort: Vincent Marconi and Jonathan Colasanti.
Fenway Health HIV Cohort: Kenneth H. Mayer and Chris Grasso.
HAART Observational Medical Evaluation and Research: Robert S. Hogg, Viviane D. Lima, Julio S. G. Montaner, Paul Sereda, and Kate Salters.
HIV Outpatient Study: Kate Buchacz and Jun Li.
HIV Research Network: Kelly A. Gebo and Richard D. Moore.
Johns Hopkins HIV Clinical Cohort: Richard D. Moore.
John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Jeffrey M. Jacobson.
Kaiser Permanente Mid-Atlantic States: Michael A. Horberg.
Kaiser Permanente Northern California: Michael J. Silverberg.
Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne.
MACS/WIHS Combined Cohort Study: Todd Brown, Phyllis Tien, and Gypsyamber D’Souza.
Maple Leaf Medical Clinic: Graham Smith, Mona Loutfy, and Meenakshi Gupta.
The McGill University Health Centre, Chronic Viral Illness Service Cohort: Marina B. Klein.
Multicenter Hemophilia Cohort Study–II: Charles Rabkin.
Ontario HIV Treatment Network Cohort Study: Abigail Kroch, Ann Burchell, Adrian Betts, and Joanne Lindsay.
Parkland/UT Southwestern Cohort: Ank Nijhawan.
Retrovirus Research Center, Universidad Central del Caribe, Bayamon Puerto Rico: Angel M. Mayor.
Southern Alberta Clinic Cohort: M. John Gill.
Study of the Consequences of the Protease Inhibitor Era: Jeffrey N. Martin.
Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy: Jun Li and John T. Brooks.
University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero, and James Willig.
University of California at San Diego: Laura Bamford and Maile Karris.
University of North Carolina at Chapel Hill HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik.
University of Washington HIV Cohort: Mari M. Kitahata and Heidi M. Crane.
Vanderbilt Comprehensive Care Clinic HIV Cohort: Timothy R. Sterling, David Haas, Peter Rebeiro, and Megan Turner.
Veterans Aging Cohort Study: Kathleen A. McGinnis and Amy C. Justice.
NA-ACCORD Study Administration:
Executive Committee: Richard D. Moore, Keri N. Althoff, Stephen J. Gange, Mari M. Kitahata, Jennifer S. Lee, Michael S. Saag, Michael A. Horberg, Marina B. Klein, Rosemary G. McKaig, and Aimee M. Freeman.
Administrative Core: Richard D. Moore, Keri N. Althoff, and Aimee M. Freeman.
Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Liz Morton, Justin McReynolds, and William B. Lober.
Epidemiology and Biostatistics Core: Stephen J. Gange, Jennifer S. Lee, Brenna Hogan, Bin You, Elizabeth Humes, Lucas Gerace, Cameron Stewart, and Sally Coburn.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the United States Department of Veterans Affairs.
Financial support. This work was supported by the National Institutes of Health (grant numbers U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050409, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01DA011602, R01DA012568, R01 AG053100, R24AI067039, R34DA045592, U01AA013566, U01AA020790, U01AI038855, U01AI038858, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01DA03629, U01DA036935, U10EY008057, U10EY008052, U10EY008067, U01HL146192, U01HL146193, U01HL146194, U01HL146201, U01HL146202, U01HL146203, U01HL146204, U01HL146205, U01HL146208, U01HL146240, U01HL146241, U01HL146242, U01HL146245, U01HL146333, U24AA020794, U54GM133807, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR002378, Z01CP010214, and Z01CP010176); the Centers for Disease Control and Prevention (contract numbers CDC-200-2006-18797 and CDC-200-2015-63931); the Agency for Healthcare Research and Quality (AHRQ) (contract number 90047713); the Health Resources and Services Administration (contract number 90051652); the Grady Health System; the Canadian Institutes of Health Research (CIHR) (grant numbers CBR-86906, CBR-94036, HCP-97105, and TGF-96118); the Ontario Ministry of Health and Long Term Care; and the government of Alberta, Canada. Additional support was provided by the National Institute of Allergy and Infectious Diseases, National Cancer Institute, National Heart, Lung, and Blood Institute, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Human Genome Research Institute, National Institute of Mental Health, National Institute on Drug Abuse, National Institute on Aging, National Institute of Dental and Craniofacial Research, National Institute of Neurological Disorders and Stroke, National Institute of Nursing Research, National Institute on Alcohol Abuse and Alcoholism, National Institute on Deafness and Other Communication Disorders, and National Institute of Diabetes and Digestive and Kidney Diseases.
Contributor Information
Kathleen A McGinnis, Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut, USA.
Amy C Justice, Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut, USA; Yale Schools of Medicine and Public Health, New Haven, Connecticut, USA.
Richard D Moore, Johns Hopkins University, Baltimore, Maryland, USA.
Michael J Silverberg, Kaiser Permanente Northern California, Oakland, California, USA.
Keri N Althoff, Johns Hopkins University, Baltimore, Maryland, USA.
Maile Karris, University of California, San Diego, San Diego, California, USA.
Viviane D Lima, University of British Columbia, Vancouver, Canada.
Heidi M Crane, University of Washington, Seattle, Washington, USA.
Michael A Horberg, Kaiser Permanente Mid-Atlantic Permanente Research Institute, Rockville, Maryland, USA.
Marina B Klein, McGill University, Montreal, Quebec, Canada.
Stephen J Gange, Johns Hopkins University, Baltimore, Maryland, USA.
Kelly A Gebo, Johns Hopkins University, Baltimore, Maryland, USA.
Angel Mayor, Universidad Central del Caribe, Bayamon, Puerto Rico, USA.
Janet P Tate, Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut, USA; Yale School of Medicine, New Haven, Connecticut, USA.
North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD)a of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) and Veterans Aging Cohort Study (VACS):
Constance A Benson, Ronald J Bosch, Gregory D Kirk, Vincent Marconi, Jonathan Colasanti, Kenneth H Mayer, Chris Grasso, Robert S Hogg, Viviane D Lima, Julio S G Montaner, Paul Sereda, Kate Salters, Kate Buchacz, Jun Li, Kelly A Gebo, Richard D Moore, Richard D Moore, Jeffrey M Jacobson, Michael A Horberg, Michael J Silverberg, Jennifer E Thorne, Todd Brown, Phyllis Tien, Gypsyamber D’Souza, Graham Smith, Mona Loutfy, Meenakshi Gupta, Marina B Klein, Charles Rabkin, Abigail Kroch, Ann Burchell, Adrian Betts, Joanne Lindsay, Ank Nijhawan, Angel M Mayor, M John Gill, Jeffrey N Martin, Jun Li, John T Brooks, Michael S Saag, Michael J Mugavero, James Willig, Laura Bamford, Maile Karris, Joseph J Eron, Sonia Napravnik, Mari M Kitahata, Heidi M Crane, Timothy R Sterling, David Haas, Peter Rebeiro, Megan Turner, Kathleen A McGinnis, Amy C Justice, Richard D Moore, Keri N Althoff, Stephen J Gange, Mari M Kitahata, Jennifer S Lee, Michael S Saag, Michael A Horberg, Marina B Klein, Rosemary G McKaig, Aimee M Freeman, Richard D Moore, Keri N Althoff, Aimee M Freeman, Mari M Kitahata, Stephen E Van Rompaey, Heidi M Crane, Liz Morton, Justin McReynolds, William B Lober, Stephen J Gange, Jennifer S Lee, Brenna Hogan, Bin You, Elizabeth Humes, Lucas Gerace, Cameron Stewart, and Sally Coburn
References
- 1. Margolis AM, Heverling H, Pham PA, Stolbach A.. A review of the toxicity of HIV medications. J Med Toxicol 2014; 10:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. May MT, Gompels M, Delpech V, et al. UK Collaborative HIV Cohort (UK CHIC) Study. . Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS 2014; 28:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Byrd KK, Hou JG, Hazen R, et al. Patient-Centered HIV Care Model Team. . Antiretroviral adherence level necessary for HIV viral suppression using real-world data. J Acquir Immune Defic Syndr 2019; 82:245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000; 133:21–30. [DOI] [PubMed] [Google Scholar]
- 5. Wong C, Gange SJ, Moore RD, et al. North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). . Multimorbidity among persons living with human immunodeficiency virus in the United States. Clin Infect Dis 2018; 66:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hogg RS, Eyawo O, Collins AB, et al. Comparative Outcomes and Service Utilization Trends (COAST) study. . Health-adjusted life expectancy in HIV-positive and HIV-negative men and women in British Columbia, Canada: a population-based observational cohort study. Lancet HIV 2017; 4:e270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park LS, Tate JP, Sigel K, et al. Association of viral suppression with lower AIDS-defining and non-AIDS-defining cancer incidence in HIV-infected veterans: a prospective cohort study. Ann Intern Med 2018; 169:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Althoff KN, McGinnis KA, Wyatt CM, et al. Veterans Aging Cohort Study (VACS). . Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis 2015; 60:627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Althoff KN, Chandran A, Zhang J, et al. North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. . Life-expectancy disparities among adults with HIV in the United States and Canada: the impact of a reduction in drug- and alcohol-related deaths using the lives saved simulation model. Am J Epidemiol 2019; 188:2097–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tate JP, Justice AC, Hughes MD, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS 2013; 27:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akgün KM, Gordon K, Pisani M, et al. Risk factors for hospitalization and medical intensive care unit (MICU) admission among HIV-infected veterans. J Acquir Immune Defic Syndr 2013; 62:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salinas JL, Rentsch C, Marconi VC, et al. Baseline, time-updated, and cumulative HIV care metrics for predicting acute myocardial infarction and all-cause mortality. Clin Infect Dis 2016; 63:1423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Womack JA, Goulet JL, Gibert C, et al. Veterans Aging Cohort Study Project Team. . Physiologic frailty and fragility fracture in HIV-infected male veterans. Clin Infect Dis 2013; 56:1498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marquine MJ, Montoya JL, Umlauf A, et al. HIV Neurobehavioral Research Program Group. . The Veterans Aging Cohort Study (VACS) Index and neurocognitive change: a longitudinal study. Clin Infect Dis 2016; 63:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marquine MJ, Umlauf A, Rooney AS, et al. HIV Neurobehavioral Research Program (HNRP) Group. . The Veterans Aging Cohort Study Index is associated with concurrent risk for neurocognitive impairment. J Acquir Immune Defic Syndr 2014; 65:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tate JP, Sterne JAC, Justice ACVeterans Aging Cohort Study (VACS) and the Antiretroviral Therapy Cohort Collaboration (ART-CC). . Albumin, white blood cell count, and body mass index improve discrimination of mortality in HIV-positive individuals. AIDS 2019; 33:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS cohort collaboration on research and design (NA-ACCORD). Int J Epidemiol 2007; 36:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care 2006; 44:S25–30. [DOI] [PubMed] [Google Scholar]
- 19. Navar AM, Peterson ED, Steen DL, et al. Evaluation of mortality data from the Social Security Administration death master file for clinical research. JAMA Cardiol 2019; 4:375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maynard C. The incompleteness of the Social Security death master file. JAMA Cardiol 2019; 4:831. [DOI] [PubMed] [Google Scholar]
- 21. Lash TL, Silliman RA.. A comparison of the National Death Index and Social Security Administration databases to ascertain vital status. Epidemiology 2001; 12:259–61. [DOI] [PubMed] [Google Scholar]
- 22. Lee H, Lim CW, Hong HP, et al. Efficacy of the APACHE II score at ICU discharge in predicting post-ICU mortality and ICU readmission in critically ill surgical patients. Anaesth Intensive Care 2015; 43:175–86. [DOI] [PubMed] [Google Scholar]
- 23. Mooney S, Tracy R, Osler T, Grace C.. Elevated biomarkers of inflammation and coagulation in patients with HIV are associated with higher Framingham and VACS risk index scores. PLoS One 2015; 10:e0144312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yuh B, Tate J, Butt AA, et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis 2015; 60:1852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sohn MW, Arnold N, Maynard C, Hynes DM.. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006; 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maynard C. Ascertaining veterans’ vital status: VA data sources for mortality ascertainment and cause of death. Available at: https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3544-notes.pdf. Accessed 23 March 2021. [Google Scholar]
- 27. Sterling RK, Lissen E, Clumeck N, et al. APRICOT Clinical Investigators. . Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–25. [DOI] [PubMed] [Google Scholar]
- 28. Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Justice AC, Tate JP.. Strengths and limitations of the Veterans Aging Cohort Study Index as a measure of physiologic frailty. AIDS Res Hum Retroviruses 2019; 35:1023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.