Figure 1.
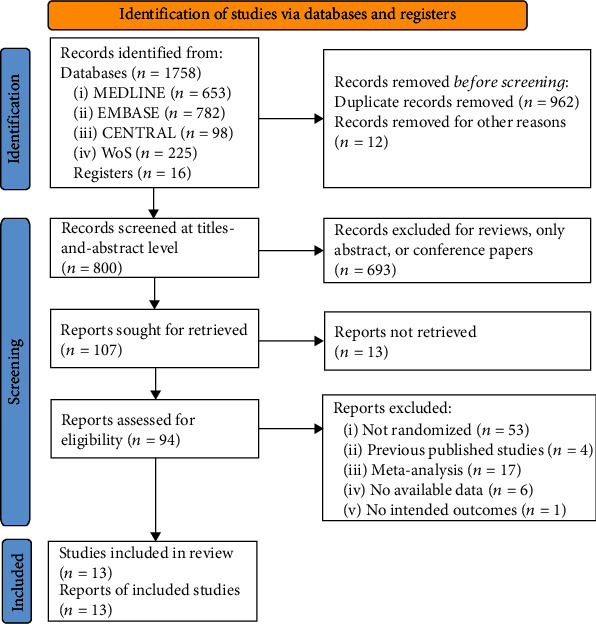
Screening process. Abbreviations: CENTRAL: Cochrane Central Register of Controlled Trials. WoS: Web of Science. Footnotes: In records removed for other reasons, the reasons included ongoing trials with only register numbers (n = 8), terminated trials with only notice (n = 3), and one article without authors and publication source. “no available data” refers to the studies reported the assessment of pain intensity or adverse events but has no relevant data to extract for analysis. “no intended outcomes” refers to the studies reported no assessment of pain intensity or adverse events.
