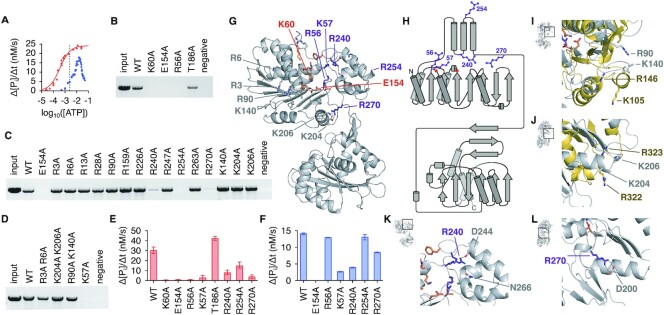
Figure 5.
Critical residues for HK97 TerL ATPase activity and DNA translocation. (A) ATP hydrolysis rates, based on inorganic phosphate release, of free TerL (blue) and TerL in the presence of DNA, TerS and procapsids (red) as a function of ATP concentration. Fitting of the Hill equation suggested a Vmax of 22.7 nM/s, Km of 0.32 mM and n of 1.4 for TerL in the DNA packaging assembly. The average physiological ATP concentration, 1.5 mM (84), is indicated with a dotted line. (B–D) DNA packaging by ATPase alanine TerL mutants. (E) ATPase activity of mutant TerL in the presence of DNA, TerS and procapsids at 1 mM ATP concentration. The mean and range of three initial rate measurements are reported for each condition. (F) ATPase activity of free mutant TerL at 10 mM ATP concentration. (G) Ribbon representation of the HK97 TerL structure with ATPase active site residues indicated in orange, and other catalytically important arginine or lysine residues purple. (H) Topology diagram with the same residues marked. Superposition of the HK97 structure with (I) the trans-acting residues of the phi29 packaging ATPase (gold, PDB 7JQQ) and (J) the arginine finger of NTP-binding domain 1 of Thermus thermophilus AAA+ ATPase ClpB (gold, PDB 1QVR). (K, L) Side-chain interactions involving residues R240 and R270, respectively.