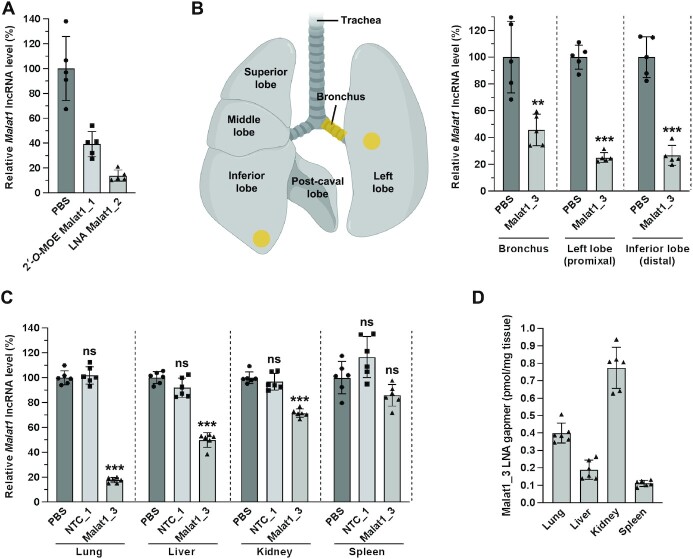
Figure 1.
Silencing efficacy in the lung and other tissues after intratracheal administration of gapmer ASOs targeting Malat1. (A, B) Mice were intratracheally administered 7.5 nmol of 2′-O-MOE ASO (∼2.2 mg kg–1), LNA ASO (∼1.5 mg kg–1), or PBS (n = 5), and lungs were collected 2 days (A) or 6 days (B) later. The silencing of Malat1 lncRNA was measured with RT-qPCR and normalized to Ppib mRNA level. Data are presented relative to the PBS group. (B) The uniformity of silencing in different regions of the lung was analyzed by collecting punches from lung regions (highlighted in yellow) before measuring RNA levels. (C, D) Mice were intratracheally administered 10 nmol (∼2.1 mg kg–1) of ASO or PBS (n = 5), and tissues were collected 1 week later. (C) The silencing of Malat1 lncRNA was measured with RT-qPCR in the lung, liver, kidney, and spleen and normalized to Ppib mRNA level. Data are presented relative to the PBS group in each tissue. (D) The concentration of Malat1 ASO in the lung, liver, kidney, and spleen was quantified by the SplintR qPCR assay. Data are presented as arithmetic mean (Mean) ± standard deviation (SD) with values of the individual animals as dots. **P < 0.01, ***P < 0.001 versus the PBS group (unpaired t-test in panel B, one-way ANOVA in panel C). 2′-O-MOE, 2′-O-methoxyethyl; LNA, locked nucleic acid; PBS, phosphate-buffered saline; NTC, non-target control. Illustration was created with BioRender.com.