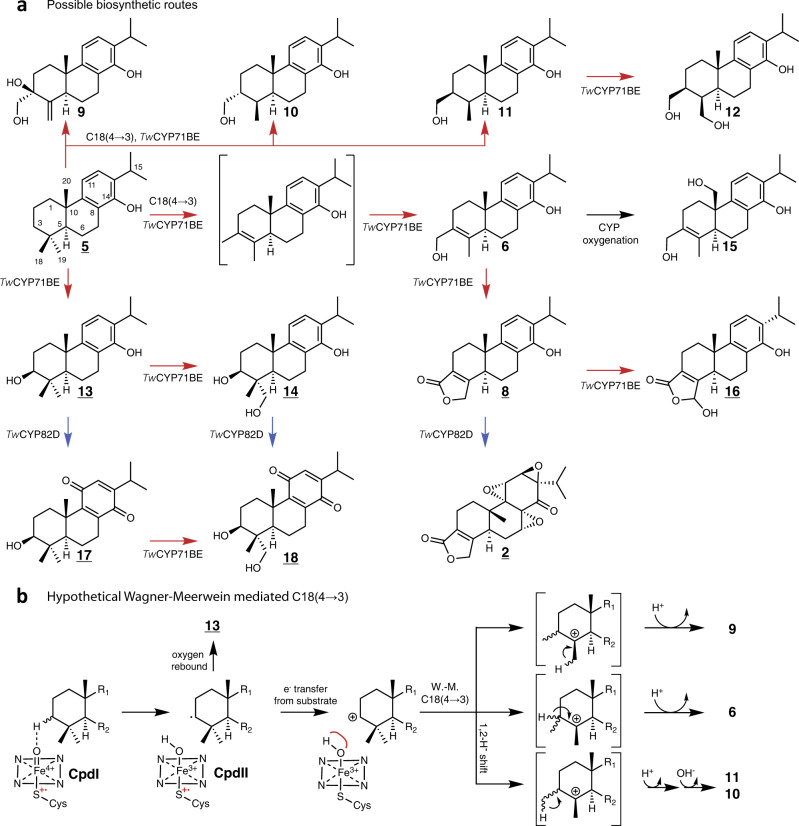
Fig. 4. Proposed biosynthetic pathway of triptonide (2) from 14-hydroxy-dehydroabietadiene (5) in S. cerevisiae.
a The biosynthetic pathway from 5 to 2 illustrating the formation of oxygenated miltiradiene derived intermediates catalyzed by the action of TwCYP82’s (blue arrows) and TwCYP71BE’s (red arrows) on the A- and C-ring of the abietane diterpene backbone, respectively. Underlined compounds or diastereomers hereof have previously been identified in plant extracts from Tripterygium species (Supplementary Table 1). b a hypothetical Wagner-Meerwein rearrangement reaction (W.-M.) to account for the methyl shift of C-18 to C-3 in the abietane carbon backbone. Cpd I and Cpd II represents states of the heme in the CYP catalytic cycle. Red line at heme bound hydroxyl symbolizes inhibition of oxygen rebound facilitating e- transfer as proposed by43 for CYP mediated carbocation formation. Compound 13 and oxygenated compounds hereof including 14, 17, and 18, would be products caused by the failure to inhibit oxygen rebound and additional oxygenation likely to be catalyzed by the TwCYPs heterolgously expressed in S. cerevisiae. Alternatively, the C18(4→3) could be mediated via a mechanism relying on the radical formed by the Compound II (CpdII) state of the involved CYP enzyme (Supplementary Fig. 7).