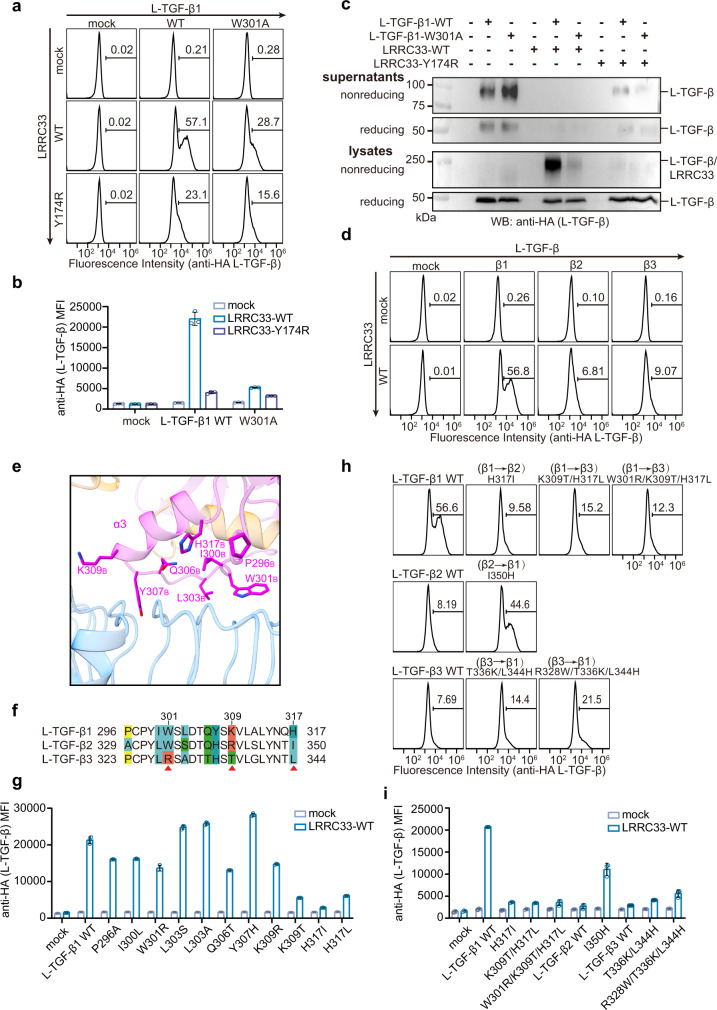
Fig. 2. Non-covalent interaction is essential for the L-TGF-β1/LRRC33 assembly and determines L-TGF-β isoform specificity.
a Surface L-TGF-β1 (anti-HA) expressions on transfected Expi293F cells were measured by flow cytometry. Number in each histogram indicates the percentage of L-TGF-β+ (anti-HA+) subset. b MFI (Mean Fluorescence Intensity) of L-TGF-β (anti-HA) on differently transfected Expi293F cells. All experiments were done in triplicate (n = 3 biologically independent experiments, mean ± s.d.). c Culture supernatants and total lysates of Expi293F cells transfected with indicated plasmids were subjected to non-reducing and reducing immunoblot analyses with anti-HA antibody (for L-TGF-β). The experiment was repeated three times independently with similar results. d Surface presentations of different L-TGF-β isoforms by LRRC33 were measured by flow cytometry. Number in each histogram indicates the percentage of L-TGF-β+ (anti-HA+) subset. e The non-covalent interface between mTGF-β1 and LRRC33. Side chains are shown for the mTGF-β1 residues that are in close distance with LRRC33. f Sequence alignment of the potential LRRC33-binding regions of the three L-TGF-β isoforms. Non-conserved residues are colored. g Surface presentation (MFI) of different L-TGF-β1 mutants by LRRC33. All experiments were done in triplicate (n = 3 biologically independent experiments, mean ± s.d.). h, i Cross mutations of the three key residues between different L-TGF-β isoforms switch their LRRC33-binding specificity. All experiments were done in triplicate (n = 3 biologically independent experiments, mean ± s.d.). Source data are provided as a Source Data file.