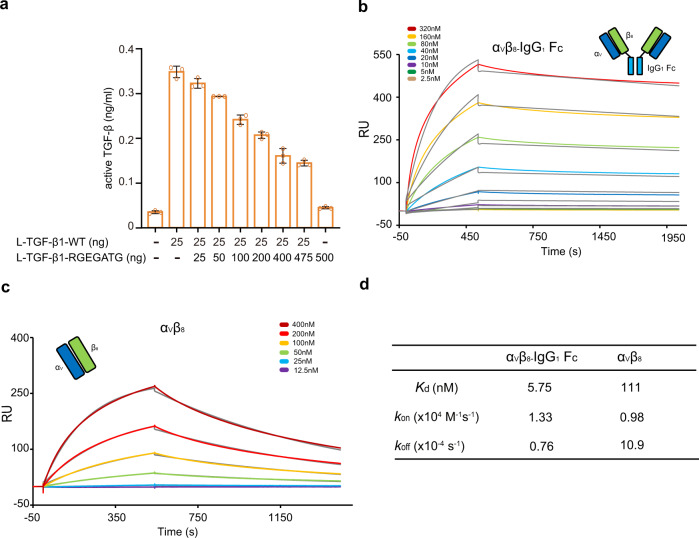
Fig. 4. Functional significance of the 2:2 binding stoichiometry between integrin αVβ8 and L-TGF-β1.
a The measured TGF-β1 activity in (CAGA)12-Luciferase reporter cells when co-cultured with L-TGF-β1/GARP and integrin αVβ8 expressing cells. The transfected amount of WT and integrin-binding defective (RGEGATG) L-TGF-β1 plasmids was indicated. All experiments were done in triplicate (n = 3 biologically independent experiments, mean ± s.d.). b, c The surface plasmon resonance (SPR) results for affinity measurements between L-TGF-β1 and two integrin αVβ8 variants. Both the experimental (chromatic) and fitting (gray) curves are shown. αVβ8-IgG1 Fc refers to a heterotetramer of αVβ8 linked by IgG1 Fc fragment. As indicated, the concentrations of integrins used in the experiment were different in the two experiments. d Summarization of the dissociation constants (Kd) and kinetic parameters (kon and koff). Source data are provided as a Source Data file.