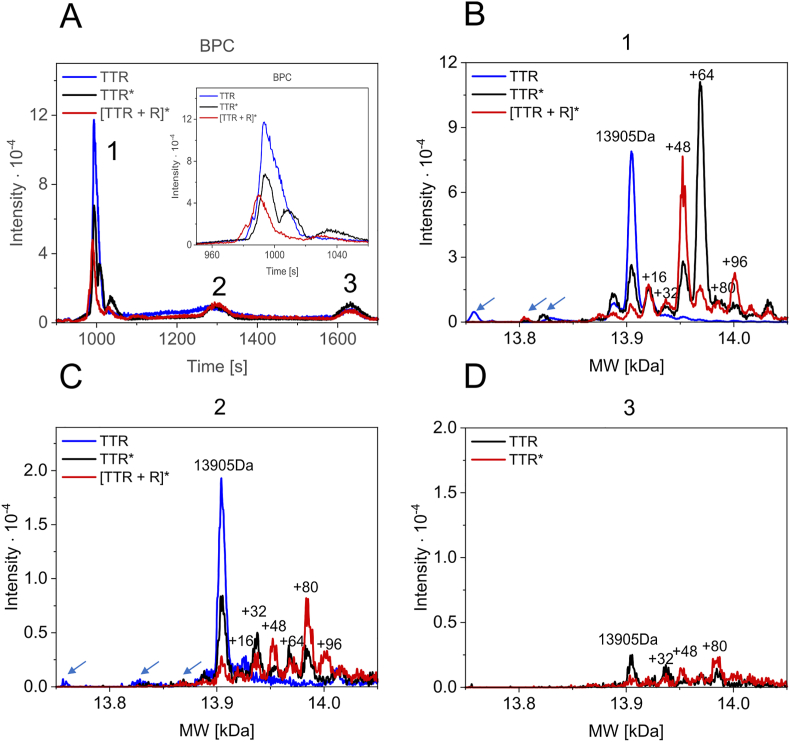
Fig. 7.
Irradiation results in the formation of multiple oxidized forms of TTR TTR samples (35 μM monomer) in HEPES buffer were preincubated in the absence or presence of riboflavin (100 μM) and subjected to irradiation at 23 °C for 30 min using an excitation wavelength of 445 nm and 2.0 nm slits. The irradiated samples were incubated overnight at 60 °C and subjected to size-exclusion chromatography on a Superdex S75 Increase column. The peak protein fractions and nonirradiated TTR (50 μM monomer) in HEPES buffer were vacuum dried, resuspended in 2% acetonitrile with 0.05% TFA and centrifuged at 21,000×g for 15 min at 4 °C. Fifty nanograms of each protein sample was separated on a 15 cm × 75 μm Accucore™ 150-C4 column (A). MS spectra were obtained for the main protein peaks. The MW determination of the protein by a deconvolution multiply charged ion series was performed using maximum entropy software. (B, C, D) The superimpositions of the protein deconvoluted spectra obtained for peaks 1, 2 and 3 of all samples. The insert in A shows the enlargement of superimposed peak no. 1.