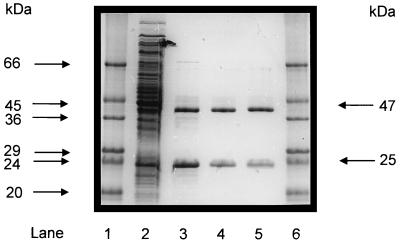
FIG. 2.
Purification of recombinant acetyl-CoA synthetase (ADP forming) after reconstitution from its subunits (α and β) separately expressed in E. coli, as analyzed by SDS-PAGE. Protein was separated on a 12% polyacrylamide gel and subsequently stained with Coomassie brilliant blue R-250 (13). Lanes 1 and 6, molecular mass standards (Sigma); lane 2, 20 μg of protein of combined cell extract (10 μg each) of E. coli transformed with either pET-14b (acdBI) or pET-17b (acdAI) vector after induction with IPTG; lane 3, 6 μg of 13,000 × g supernatant of combined E. coli extracts (see lane 2) after heat treatment (15 min, 80°C); lane 4, 2.5 μg of purified recombinant enzyme after chromatography on Resource Q; lane 5, 2.5 μg of native acetyl-CoA synthetase (ADP forming) purified from P. furiosus (7).