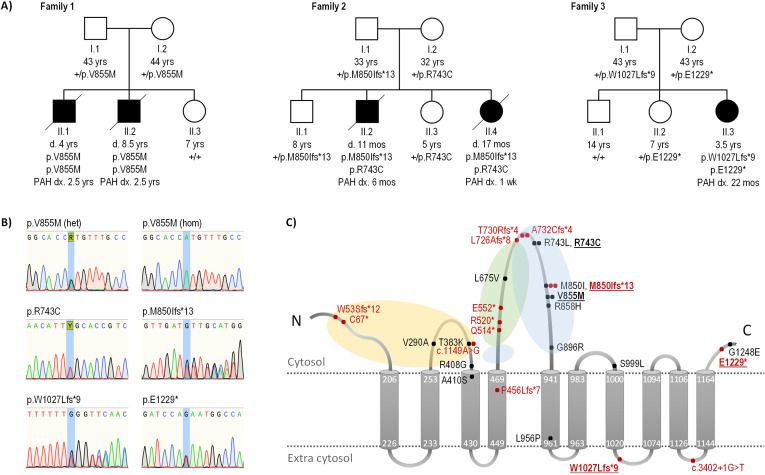
Figure 1.
Rare deleterious ATP13A3 variants in biallelic and monoallelic PAH. (A) Family pedigrees for cases with early-onset, severe biallelic PAH. Filled boxes and circles indicate affected individuals. ATP13A3 mutation status is detailed for each individual. (B) Sequence chromatograms illustrating identified ATP13A3 variants in families 1–3. (C) ATP13A3 topology model with locations of variants identified in all PAH cases, both novel (bold typeface) and previously reported (Graf 2018, Zhu 2019, Wang, 2019, Lerche 2019, Gelinas 2020). Missense variants are in black, predicted truncating/splice variants in red. The number of filled circles at a variant location indicates the number of unrelated PAH cases identified with the variant. Yellow, green and blue shaded areas represent the actuator, nucleotide-binding and phosphorylation domains, respectively. Amino acids are numbered according to the longest transcript (NM_001367549.1; NP_001354478.1). +, wild type allele; d., age at death; dx., age at diagnosis; het, heterozygous; hom, homozygous; mos, months; PAH, pulmonary arterial hypertension.