Figure 2. Overall Survival (OS) Associated With First-Line Immune Checkpoint Inhibitor (ICI) Therapy vs Targeted Therapy Among Patients With Stage IV Melanoma Diagnosed Following US Food and Drug Administration (FDA) Approval of First-Line Anti–PD-1 in Late 2015.
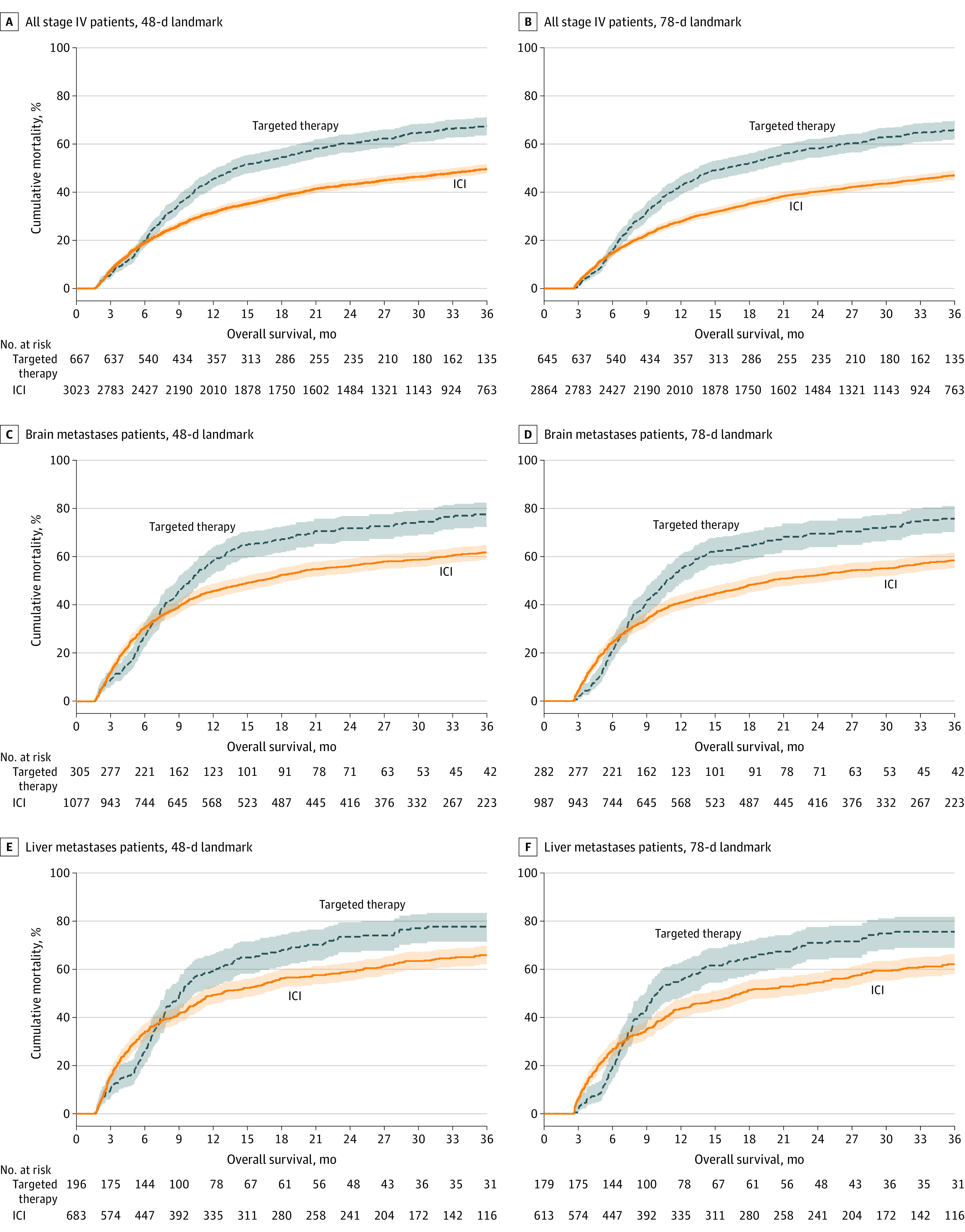
OS was estimated and plotted using Kaplan-Meier techniques with corresponding 95% CIs for patients that received first-line ICI therapy (orange) and those that only received first-line non-immunotherapeutic systemic therapy (blue). OS was assessed for patients diagnosed in 2016 through 2018. Survival outcomes were not reported for patients diagnosed in the last year of the data set, which was 2019 herein. To account for immortal time bias associated with receipt of ICI, landmark time points were used corresponding to the 50th percentile (ie, 48 days; A, C, E) and 75th percentile (ie, 78 days; B, D, F) of time from diagnosis to ICI initiation.
