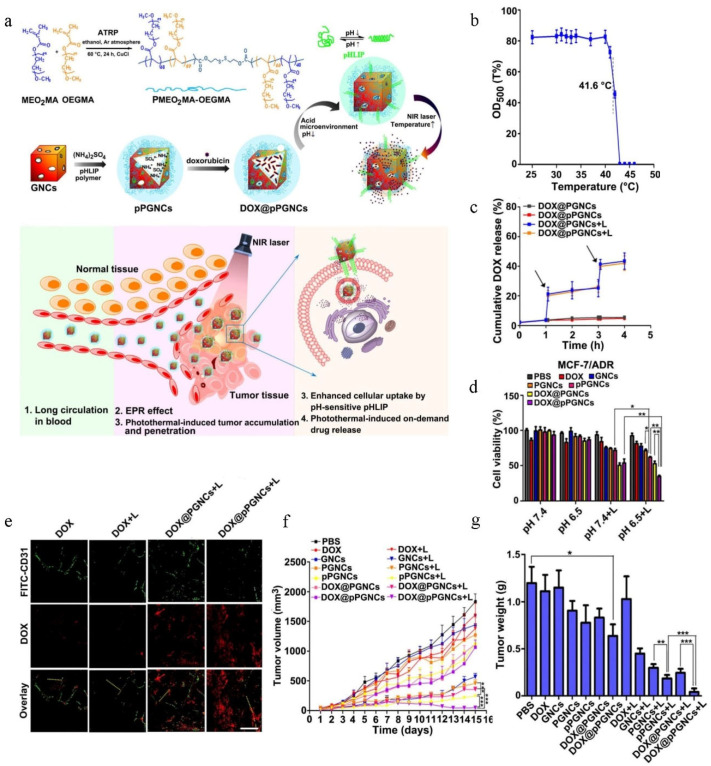
Figure 3.
DOX@pPGNCs for chemo-photodynamic combination therapy of drug-resistant cancer. Reprinted with permission from Ref. [99]. 2019, NIH. (a) Schematic diagram of the mechanism of DOX@pPGNCs surmounting cancer drug resistance. (b) Transmittance of POEG in PBS. (c) In vitro release curve of DOX@pPGNCs. (d) In vitro cytotoxicity against MCF-7/ADR cells. * p < 0.05, ** p < 0.01. (e) Accumulation of different groups in tumor site of tumor-bearing mice. (f,g) In vivo antitumor effect of DOX@pPGNCs. * p < 0.05, ** p < 0.01, *** p < 0.001. P-gp, an ATP-dependent protein, is overexpressed in different drug-resistant tumor cells and closely related to chemotherapy resistance by promoting drug efflux [100,101,102,103,104]. In recent few years, RNA interference (RNAi) techniques have been used to block the expression of P-gp to reverse drug resistance [105,106,107,108,109]. Zheng et al. [109] constructed a special, siRNA and chemotherapy drug co-delivery system, an siRNA-based nanostructure (siRNAsome), to enhance combination therapy for overcome chemotherapeutic resistance (Figure 4a). Dynamic light scattering (DLS) data show that Dox.HCl-loaded siRNAsome (Pgp-siRNAsome@Dox.HCl) ranged in particle size from 126 to 135 nm. This novel siRNAsome was based on the self-assembly of siRNA-disulfide-poly (N-isopropylacrylamide) (siRNA-SS-PNIPAM) copolymers, which was very different from traditional siRNA delivery systems. In other words, this distinctive siRNAsome not only possessed an empty aqueous interior that could load hydrophilic agents, but also possessed a thermoresponsive and intracellular reduction-dependent hydrophobic median layer that could load hydrophobic drugs. Moreover, this siRNAsome possessed a siRNA stabilization shell that could load siRNA drugs without using a cationic component. When siRNAsome was incubated with a dithiothreitol (DTT) solution, DOX was rapidly released from the nanostructure, and more than 75% of the encapsulated DOX was released after 24 h incubation with dithiothreitol (Figure 4b), indicating that siRNAsome was sensitive to an intracellular environment and intracellular redox conditions could effectively disintegrate the structure of siRNAsome to control drug release. To test the capacity to efficiently deliver siRNA into tumor cells without the aid of a cationic component, MCF-7/ADR cells were incubated with siRNAsome and confocal laser scanning microscopy showed that siRNAsome could unquestionably promote uptake of siRNAsome (Figure 4c). More importantly, when treated with siRNAsome for 2 days, the P-gp mRNA level of MCF-7/ADR cells decreased by approximately 42% by P-gp gene silencing (Figure 4d). To test the synergistic antitumor effect of the siRNAsome, DOX and anti-P-gp siRNA were co-loaded into the siRNAsome to form Pgp-siRNAsome@Dox.HCl. As shown in Figure 4e,f, Pgp-siRNAsome@Dox.HCl showed the strongest cytotoxicity in MCF-7/ADR cancer cells and the strongest antitumor effect in MCF-7/ADR cell-bearing mice, indicating that the knockdown of P-gp mRNA could remarkably improve the activity of DOX to efficiently realize synergistic therapeutic efficacy and this cation-free Pgp-siRNAsome@Dox.HCl nanostructure could serve as a promising vehicle for reversing drug resistance.