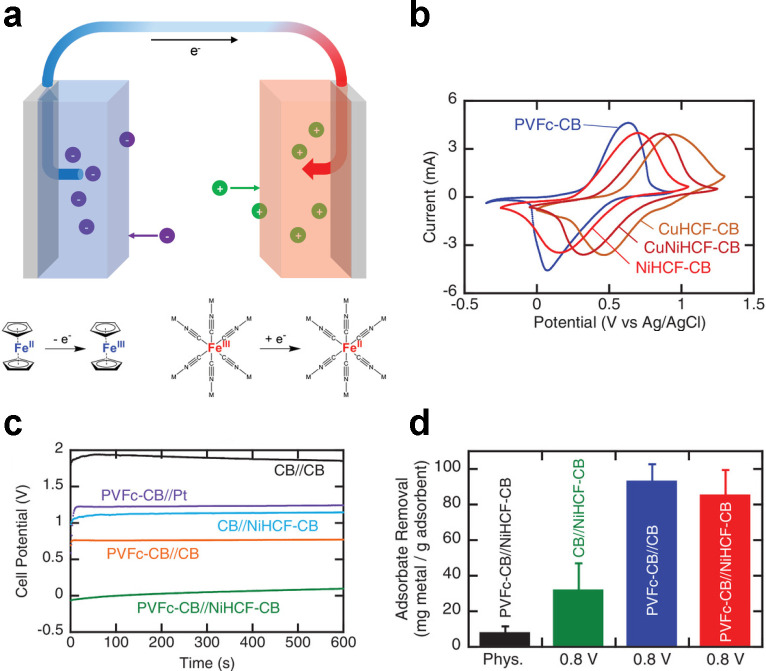
Figure 35.
Asymmetric redox system using iron active centers for selective separations. (a) Schematic of the asymmetric Faradaic cell, in which a ferrocene metallopolymer is oxidized at the anode (blue) and an HCF crystal is reduced at the cathode (red). (b) Cyclic voltammetry shows that the HCF electrode can be structurally tuned to control the redox potential. (c) Cell potential during electrosorption and (d) recovery of molybdenum anions for different electrode chemistries. Reproduced with permission from ref (985). Copyright 2020 Wiley-VCH.