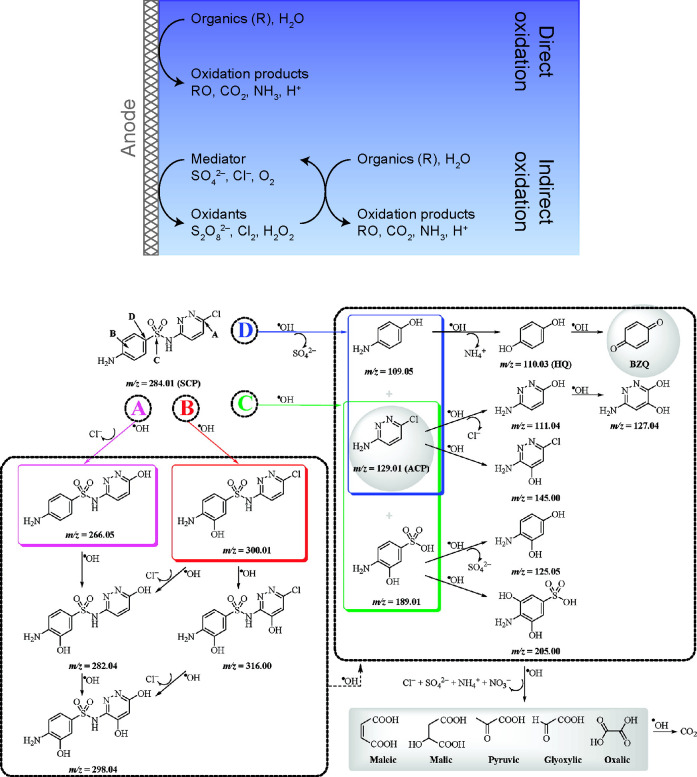
Figure 5.
Electrochemical oxidation is an established destructive, nonelectrosorptive method that is used to degrade most organic contaminants and certain inorganic compounds. (top) The method involves formation of reactive oxidizers that interact with contaminants either at the anode surface (direct oxidation) or in the bulk (indirect, or mediated, oxidation).123 Reproduced with permission from ref (191). Copyright 2017 Royal Society of Chemistry. (bottom) Complex organic compounds, such as the sulfonamide antibiotic sulfachloropyridazine, are degraded and mineralized by the attack of reactive oxidizers at key reaction sites (designated by the letters A–D). In this example, hydroxyl radicals can attack four unique sites on sulfachloropyridazine to yield different primary cyclic byproducts, all of which are eventually transformed into CO2. Reproduced with permission from ref (192). Copyright 2012 American Chemical Society.