Abstract
Substandard and falsified (SF) medicines are a global health challenge with the World Health Organization (WHO) estimating that 1 in 10 of medicines in low- and middle-income countries (LMICs) are SF. Antimicrobials (i.e. antimalarials, antibiotics) are the most commonly reported SF medicines. SF medicines contribute significantly to the global burden of infectious diseases and antimicrobial resistance (AMR). This article discusses the challenges associated with the global impact of SF and unregistered/unlicensed antimicrobials with a focus on anti-TB medicines. Tuberculosis (TB) is the 13th leading cause of death worldwide, and is currently the second leading cause of death from a single infectious agent, ranking after COVID-19 and above HIV/AIDS. Specifically in the case of TB, poor quality of anti-TB medicines is among the drivers of the emergence of drug-resistant TB pathogens. In this article, we highlight and discuss challenges including the emergence of SF associated AMR, patient mistrust and lack of relevant data. We also present study reports to inform meaningful change. Recommended solutions involve the adaptation of interventions from high-income countries (HICs) to LMICS, the need for improvement in the uptake of medication authentication tools in LMICs, increased stewardship, and the need for global and regional multidisciplinary legal and policy cooperation, resulting in improved legal sanctions.
Keywords: Substandard, falsified, unregistered, unlicensed, medicines, tuberculosis, drug resistance, antimicrobial resistance
Introduction
Tuberculosis (TB) is an infectious disease affecting both humans and animals. The causative agents of TB are Mycobacterium tuberculosis in humans, primates and guinea pigs and Mycobacterium bovis in cattle, rabbits and cats. 1 Swine and dogs are susceptible to both M. bovis and M. tuberculosis. 1 According to the World Health Organization (WHO) 2021 global TB report, TB killed about 1.3 million people. 2 This is the second highest number of deaths resulting from a disease initiated by a single pathogen. 2 Depending on the region, the prevalence of bovine TB can range from 0.1% to 50%; there were 140,000 new cases in 2019, with most animals slaughtered upon discovery of an infection.3–7
The increasing proximity between humans, livestock and wildlife, and its role in the transmission dynamics of mycobacterial infections, necessitates the need for a One Health approach in tackling TB infections. The One Health approach is an integrated multidisciplinary approach to attain optimal health for humans, animals and the environment in the surveillance of zoonotic diseases such as TB. 8 The emergence of COVID-19 as a zoonotic disease, 9 and its global burden in only 2 years (more than 260 million cases and nearly 5.2 million deaths as of 3 December 2021), 10 must serve as a stellar warning for the urgent need for an integrative approach in tackling infectious diseases, with TB taking a toll of more than 1 million lives per year.
In humans, M. tuberculosis is spread through inhalation of small droplets from coughs or sneezes of an infected individual.2,7 ‘Latent’ TB infection and active TB disease are two subcategories of human TB. Latent TB is defined as a state of persistent immune response to stimulation by M. tuberculosis antigens without evidence of clinically manifested active TB. 11 Active TB is characterised by the presence of TB disease as a result of M. tuberculosis infection 7 and thus requires detection of the pathogen. TB disease is characterised by symptoms including a persistent cough, weight loss and night sweats. 12 A quarter of the world’s population has latent TB infection with about 10% being at risk of progressing to active disease. 2 In addition, it is estimated that every year 10 million people develop active TB disease.2,7 Eight low- and middle-income countries (LMICs) account for two-thirds of the global incidence of TB. Ranked from the highest to the lowest incidence rates, they include India, China, Indonesia, The Philippines, Pakistan, Nigeria, Bangladesh and South Africa. 2
The WHO recommendation for newly diagnosed TB patients with no drug resistance involves two phases: the initial phase with the use of rifampicin, ethambutol hydrochloride, pyrazinamide and isoniazid (with pyridoxine hydrochloride), and then the continuation phase using the two drugs, rifampicin and isoniazid (with pyridoxine hydrochloride). It is important to note that the mentioned TB drugs (i.e. ethambutol, Isoniazid, rifampicin and pyrazinamide) are among the WHO’s list of essential medicines, prioritised for sufficient supply, affordability and access by the general population. 13 The treatment regimen for drug-resistant TB varies depending on the type of resistance. In cases of isoniazid resistance TB, the treatment regime includes rifampicin, pyrazinamide, ethambutol and levofloxacin for 6 months and longer regimens are recommended in other forms of drug resistance. 14
The WHO defines substandard (also referred to as out of specification) medicinal products as authorised medicinal products that have failed to pass their quality standards and/or specifications.15,16 Falsified medical products are products that have been intentionally mislabelled to misrepresent their composition or source.15,16 These are different to unregistered or unlicensed medical products, which are substances that are without approval and/or necessary evaluation by the regional or national regulatory authority for the market where they are used, distributed or sold 16 Substandard and falsified (SF) medicinal products is the agreed simplified terminology used and will be used in this article moving forward. This commentary article focuses on the impact of anti-TB SF medicines, highlights their associated issues and offers solution-focused recommendations.
A total of 1 in 10 medicines in LMICs are SF. 17 Anti-infective agents such as antimalarials (e.g. chloroquine, artemisinin-based combination therapies (ACTs)) and antibiotics such as anti TB medicines (e.g. isoniazid and rifampicin) are commonly reported as being SF.17,18 The main concern with SF anti-infectives is the impact of sub-therapeutic levels of the anti-infective (or their lack of effect) resulting in prolonged infection as well as contributing to antimicrobial resistance (AMR). Patients also have a false sense of security stemming from their expectation that the medical treatment received should work according to its intended purpose. It is important to note that heterogeneity does exist in this expectation, including patient location, confidence in healthcare institutions, mental and social conditioning. 19
AMR is defined by microbial (bacteria, fungi, virus) changes that render medications ineffective and unable to cure infections. 20 AMR is a serious global health issue and threat that prevents the effective treatment of microbial infections. 21 Anti-infectives are to be used only when indicated, for the appropriate time and dose. 22 This is the underlying principle of the globally accepted TB directly observed treatment (DOTS): to enable safe and reliable treatment against TB. Anti-TB SF drugs, therefore, undermine the DOTS standard. 23 Their high representation among commonly SF drugs, similar to antimalarials, may also be reflected in TB drug resistance. The different forms of drug-resistant TB result in significantly longer treatment times and fatality rates of up to 80%, 24 making TB a global threat prioritised by the WHO.7,25 In 2014, there were an estimated 700,000 deaths that occurred due to AMR and more than one-third of these were attributed to TB patients. 24
Factors surrounding TB disease such as poor awareness and perception of the impact of the disease as well as associated mental health and psychiatric impacts make patients particularly vulnerable and more likely to be significantly impacted by anti-TB SF drugs. This is especially stark in low-resource regions situated in LMICs,26,27 which also have the highest rates of TB. 2 As a comparison, studies on malaria perception have reported a significant awareness, even in low-resource settings, that the disease is caused by mosquitoes, dirty or stagnant water creates a breeding ground. Thus, mosquito nets, and insecticides are suitable preventive measures against malaria.28–30 In stark contrast to malaria, several studies have reported a gap in the depth of awareness for TB of what is necessary for infection evasion and preventability. Community beliefs that TB occurs due to bewitchment and curses have been reported in studies conducted in several other countries, including Ghana, 31 Ethiopia,32,33 Rwanda 34 and Uganda. 35 Such beliefs have reported to impact marriage prospects, resulted in divorce, discrimination, forceful isolation of disease sufferers and withdrawal from society.26,36 These misconceptions have been shown to affect people’s perception of their risk as well as adherence to treatment or preventive measures.26,37 In addition, initial TB symptoms of cough and cold are often overlooked, and wrongly attributed to the common cold. 38 This results in further delay in seeking appropriate healthcare and relying on self-medicating (mostly with traditional healers or local drug dispensaries) and in some cases until the individual’s health significantly deteriorates.38,39
TB patients have wrongly reported to have psychosocial and psychiatric disorders, most likely stemming from the poor perception of the disease, stigmatisation and even treatment-adverse reactions.27,40–42 Early researchers believed that the presence of mental illness creates a solid predisposition to TB and vice versa. 43 Researchers and healthcare providers have reported various TB-associated psychiatric illnesses, including loss of interest in life, depression, psychosis, denial and anxiety.27,44 Poor perception, stigmatisation and TB psychopathology contribute to delay in seeking modern healthcare, loss of income and poor treatment adherence.45–48 The latter is a significant factor in the development of drug resistance.49,50 All these contribute to disease prevalence, increase patient expenses, and reduce the ability to obtain or perceived need for quality medicines or healthcare, making patients vulnerable to anti-TB SF medicines.51,52
The emergence of drug resistance
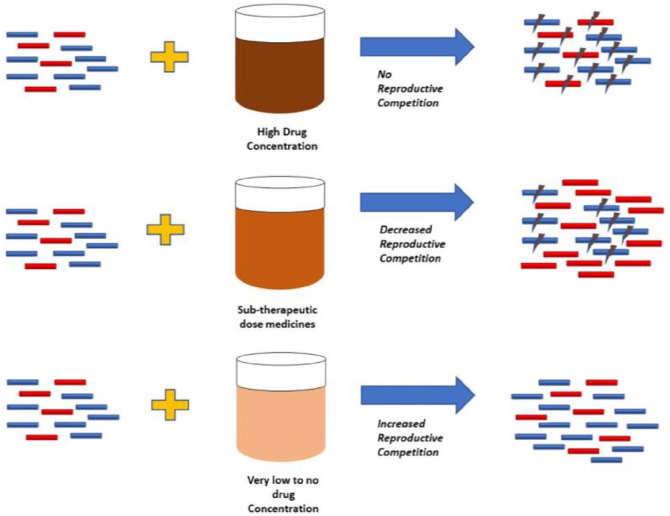
Of concern is the contribution of SFs to AMR and overall impact on the prevalence of infectious diseases. Mutations are an inherent component of microorganismal proliferation53–55 as DNA replication during cell division can accumulate mutations, that is, changes in the nucleotide sequence. Polymerases have error rates, and thus with each round of replication mutations will accumulate. 54 This may result in mutant generations with reduced susceptibility to medicines56,57 if those errors occur in processes that are the medicine’s targets. In a background of external pressure, such as that of low-efficient antimicrobial substances, mutant organisms that present a resistance gene will have a competitive advantage over those that do not have the specific mutation, resulting in reproductive competition ability that will affect drug susceptibility. 58
High concentrations of the active pharmaceutical ingredient (API) during the early stages of infection kill susceptible microbes and may kill partially resistant mutants. 59 However, SF medicines containing sub-therapeutic concentrations of the anti-infective may not be effective to achieve this, and thus mutant microorganisms will overproliferate while ‘wild type’ ones will perish. 59 Therefore, resistant SFs (rSFs) can reduce the reproduction competition and allow for the proliferation of resistant microbes. This phenomenon is summarised in Figure 1.
Figure 1.
Development of drug resistance depending on drug concentration of active agent.
Adapted from Pisani. 60
Key: Drug – susceptible bacteria indicated by blue rods, resistant bacteria indicted by red rods. The lightning sign describes death.
There are two major forms of drug-resistant TB: multi-drug resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB). MDR TB is defined as resistance to first-line TB medicines, that is, rifampicin and isoniazid. 61 Both Isoniazid and rifampicin drug resistance arises due to mutations of genes encoding proteins that are essential in the targeted biological pathways. Resistance to isoniazid arises through bacterial mutations necessary for the blocking of the synthesis of mycolic acid activity such as katG and inhA.62,63 Conversely, resistance to rifampicin occurs through mutations of the rpoB gene which codes for M.TB RNA polymerase. 64 XDR-TB occurs where MDR-TB is already present with additional resistance to fluoroquinolones and at least one of the three injectable second-line drugs, namely amikacin, kanamycin or capreomycin. 65 Fluoroquinolone drug resistance can develop through mutations in the type II topoisomerase (DNA gyrase) gene. 66 In addition, M. tuberculosis has also been shown to evolve active pumps against fluoroquinolones. 67 Resistance to the second-line injectable drugs differs and is strongly dependent on their mechanism of action.68–70
Patients receiving lower therapeutic doses of anti-TB drugs are at risk of developing drug resistance and resultant treatment failure.71,72 Bate et al. 73 performed quality control tests on 713 samples of isoniazid and rifampicin sourced from local pharmacies in 17 WHO regions, and found approximately 9% (65) of the samples were SF drugs with half of them possessing 10% to 80% isoniazid or rifampicin.
Medicine costs and distrust
The economic impact and financial loss due to SF medicines in LMICs is estimated to be US$30.5 billion annually. 17 This economic-financial cost includes several factors such as wasted human efforts and resources, economic loss for patients, their families and manufacturers of quality drugs. 17 Patients lose income due to prolonged illness, with additional expenses incurred due to drug toxicity healthcare needs, failure of the treatment or even premature death. 17
In LMICs, anti-TB SF drugs can contribute to an already existing loss of trust or confidence in the government and healthcare systems by the public. 74 A 2011 systematic review by Berendes et al. 74 suggested that poor perception of the health system, especially relating to the clinical skills and technical competence of clinicians, exists in LMICs. Qualitative research in China found that patients viewed the private health care system negatively, with references to ‘falsified doctors’ and ‘falsified drugs’. 75 SF medicines are to worsen this already existing negative perception. Public health systems, including healthcare agencies and health practitioners, can also lose confidence about the effectiveness of TB medications, due to reports of drug resistance arising from SF drug use. As a result, pharmaceutical companies and health institutions invest in the research and discovery of new replacement agents. DiMasi et al., 76 showed that the cost of drug development grows faster than inflation by 7.4%. The estimated cost for the development of a single drug is US$2.6 billion. 77 Even with high capital, antibiotics including anti-TB medicines offer reduced return on investment as their intake is limited, unlike other chronic disease drugs that are lifetime medications. Consequently, there is a low financial incentive as most of the consumers are in the poorest parts of the world.24,78
Lack of documentation of rates or study reports on TB SFs
There is limited information on the actual adverse effects or death rates for TB due to SF medicines. This is in stark contrast to other diseases such as malaria or pneumonia. In sub-Saharan Africa, SF antimalarials are thought to cause 267,000 additional deaths, which is more than half of the total global malarial deaths per year. 17 Taking into consideration the significantly higher fatality rates of TB (16%), compared with malaria (2%) and the already existing drug resistance issues, it appears safe to consider that SF drugs may be contributing to TB fatality more than believed. 79 Anti-TB SF medicines containing rifampicin and pyrazinamide can contain excessive amounts of active agents, rather than the usual insufficient or absent active drug. 80 Accidental overdose of TB drugs including rifampicin may cause severe adverse events (red man syndrome) or death.81,82 Unfortunately, there is a gap in the literature on the actual rates and incidence of excessive dosing by anti-TB SF medicines. This issue has also been highlighted by other studies.83,84 The WHO states an urgent need to obtain reliable data, particularly from sufficient sample sizes to aid reliability of the estimates of the impact and prevalence of anti-TB SF overdose.17,83,84
Several factors contribute to death by anti-TB SF medicines. First, there is invisibility that surrounds TB disease. When the disease affected high-income countries (HICs), it was at the forefront of public interest.85,86 However, due to better living conditions and access to quality healthcare, it seems like a forgotten plague. 87 TB is now associated with poverty, affecting the most economically disadvantaged and people in low-resource rural regions.88,89 As such, although it continues to kill millions of people each year,2,7 TB remains invisible. Furthermore, the association with poverty, cost of diagnosis and treatment of TB necessitates improved access to anti-TB drugs in high-burden settings.90–92 Hence, the disease is heavily reliant on generic medicines.90,93 Ongoing debates on the negative impact of anti-counterfeiting measures on the (legitimate) generic medicines industry have caused distraction and resulted in delayed assessment of anti-TB SF-related issues.94,95 There is an urgent need for further rigorous in country-led research in anti-TB SF. Standardisation of medicine quality studies using methods such as the Medicine Quality Assessment Reporting Guidelines (MEDQUARG) may improve the quality of studies conducted and create homogeneousness. 96 Theoretically, this approach could allow for comparability of studies across global regions. However, standardisation may also disadvantage regions that do not have the capacity (e.g. financial and logistical) nor consider or accommodate the heterogeneity within the individual regions. This will impact testing strategies, sampling and resource capacity. 97 An updated guideline as suggested by McManus and Naughton, 97 which allows the inclusion of contextual differences when reporting these medical quality studies, may help alleviate this issue.
Recommended solutions
There is no one simple solution to the issue of SF drugs, specifically anti-TB SF medicines. Due to the limited data available as well as a few successful examples in the literature, some of the recommendations provided here are based on the author’s recommendations and not on reported work.
Adaptation of interventions from HICs to LMICs
Although global harmonisation is constantly recommended, regional policy development and implementation may be more feasible and realistic. Policies and guidelines introduced at a global scale are recommended by HICs and this ‘one style fits all’ approach is not applicable in LMICs. This is confirmed by the difficulty faced by LMICs when it comes to implementing certain guidelines such as the WHO’s good manufacturing practices. 98 Hence, other interventions must be appropriately adjusted and applied for implementation in LMICs. These include improvement of legal sanctions, increased stewardship, advocacy and regional cooperation in the implementation of international guidelines. The latter include medicine serialisation laws similar to the EU Falsified Medicines Directive (EU FMD) and The U.S. Drug Supply Chain Security Act (DSCSA).99,100 Medicines serialisation provides further data and detailed information on drug location and drug manufacturer throughout the supply chain.101,102 Adopting laws such as these will ensure manufacturers’ mandatory implementation of medicines serialisation technology and the traceability of drugs, especially in LMICs.99,100
It is important to note that drug authentication technologies, mainly through product verification, already exist in multiple LMICs, with apparent success. Notably, Nigeria’s National Agency for Food and Drug Administration and Control (NAFDAC) unique drug code allows the identification of legit drugs and has led to a significant reduction of counterfeit medicines from 40% in 2001 to 16.7% in 2015. 103 However, there still exists a deficiency in data, visibility, distribution and illegitimate drug control gap that medicines serialisation technology can fill. 104 These data can be leveraged to ascertain the specific location where an illegitimate drug entered the supply chain, allowing for precision and rapid recall. In addition, it can provide data on the incidence of falsified medicines, which is currently lacking and necessary for quantifying the problem, especially in LMICs. 105 Hence, mandatory drug serialisation combined with authentication has the potential to bridge the gap of fake medicines detection standards of LMICs compared with HICs.
LMICs face different challenges in the implementation of serialisation technology. An important area is the scarcity of expertise and skilled personnel. 106 Others include financials, logistics, technology/infrastructure, data management challenges and productivity/cost issues. 107 Therefore to address these, actions such as investments in local skill development, 106 either through know-how transfer by foreign experts/investors or skills training of existing workers, is essential. There needs to be a safe data infrastructure that allows for safe exchange of knowledge and tracking of medicines; while its management and access has to be local, it must allow for safe communication and exchange of information between regions and countries. In addition, fiscal or financial incentives can be introduced to allow sustainability and encourage implementation. 108 For example, Turkey’s re-reimbursement of pharmacists for verified dispensing and prescriptions have been cited as significant to the success of the country’s drug track and trace system.107,108 Also, governmental investments in infrastructure such as ensuring optimal power and electricity supply to aid communications, digital operations and data management are necessary. Innovative approaches to this, including green and solar energy, may aid implementation, especially in low-resource settings. 109 Finally, implementing a regional strategy where national hubs exist within individual countries. However, reporting from each region (e.g. West Africa) to a regional-level management authority 110 may facilitate implementation in LMICs. This approach will enable the joining of resources by individual countries and reduce the burden of the challenges mentioned.
Improved update of medication authentication tools in LMICs
Detection tools for infield testing of suspected SF drugs by evaluating API content are readily available. 111 However, greater uptake and evaluation of usability in different settings in LMICs is required. For example, the technology firm Sproxil has taken a mass ‘track and trace’ or serialisation approach where a patient can scratch off a panel on the product at the point of purchase to reveal a code which is sent by text message to confirm whether the product is genuine. 112 This approach has been effective with more than 28 million verifications and collaborations with multinationals such as GlaxoSmithKline. 113 However, this solution will not work in settings where there is poor reception or poor understanding/trust on the technology itself (i.e. the person feeling that they are being tracked). With the impact of anti-TB SF medicines on TB drug level (i.e. sub-therapeutic levels resulting in drug resistance and excessive levels resulting in overdose with resultant adverse effects), implementing in field drug content testing as part of normal practice could prove beneficial. These approaches cannot be ‘one size fits all’ either, and must consider not only the technology itself but differences in culture, perception and feasibility of implementation. In some settings, having a person that ‘validates’ the purchased medicine may work better than allowing the users to do it themselves.
Increased stewardship and advocacy
A multi-stakeholder advocacy and awareness strategy against the global impact of SF medicinal products is important. These can be done through television adverts, signages or posters providing palatable information for the public on how to identify SF medicines. This can then be specifically tailored for therapeutic agents of particular concern in the specific region, for example, anti-TB or antimalarial medicines in LMICs. SF medicines should be added as a specific area of advocacy in TB stewardship strategies. Importantly, awareness of SFs in the community can also start from school, so that children can learn that this exists and poses a real problem. There also exists a gap regarding the depth of knowledge of TB disease.26,37 As such, a different approach to awareness and education is important. Emphasis should be placed on disease education as it relates to the origin and causal agent of TB as well as better prognosis when modern healthcare services are utilised. This will make people better equipped at decision making regarding prevention, treatment and social measures. Understanding of TB, its consequences as well as the damage that anti-TB SF can cause are paramount for their rejection and reporting.
Improvement of legal sanctions
Given that SF drugs cause financial loss, hospitalisation and fatalities, the penalties given should reflect these effects. The WHO has stressed that little or non-existent legislation for severe punishments for drug falsifying is a problem. 114 Currently, the penalties for drug falsifiers in Nigeria is a fine of ₦500,000 (US$1200 (as of 2021)) and/or 5 to (*greater than*) 15 years in prison. 115 Petty criminals see drug falsifying as a way of making a lot of money with lower penalties in comparison to cocaine, heroin or crack trade. 116 Therefore, drug falsifiers gravitate towards countries with weak healthcare regulatory legislature and legal laws. Improving the legal sanctions, via regional and international cooperation, is paramount in stopping anti-TB SFs.
Global and regional multidisciplinary legal and policy cooperation
An example of cooperation between different sectors has been seen in Rwanda where border customs and the ministry of health work together to ensure inspection of each shipment of TB drugs upon importation. Where SF drugs are found, they partner with the Rwandan police to charge and arrest the persons or criminals responsible. 117 Rwanda’s TB rates have also significantly declined over the years and it recorded over 85% success rates in drug-resistant TB treatment. 118 Nonetheless, the Rwandan government is aware of the necessity of working together with neighbouring countries. As such the Rwandan government and other East African countries have drafted a regional law of a unified plan against SF medicines. 117 They also recently reviewed their laws to be in line with the African Union model law on medicine regulation which facilitates the harmonisation of efforts. 119
Conclusion
The problem of anti-TB SF medicines cannot be eradicated by the solo action of an institution or country, or individual stakeholders. Therefore, different sectors within and outside of countries or regions must cooperate and work together unanimously. The extent of damage SF medicine has on TB control needs to be acknowledged and reported. While there is information on the presence of anti-TB SF medicines in LMICs, there are no studies on the effect of SF drugs on resistance development problems and their contribution to the mortality rates of the disease. This is an area that requires urgent addressing to avoid further spread of resistant TB and to tackle the problem in these areas.
Anti-TB SF drugs are an economic and significant contributor to the persistence of the disease. It is time for the TB community to recognise and quantify the damage that is occurring, and to support the identification and implementation of interventions.
Acknowledgments
The graphical abstract was created using Biorender.com.
Footnotes
Author contributions: Tamara Akpobolokemi: Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Rocio Teresa, Martinez-Nunez: Supervision; Writing – review & editing.
Bahijja Raimi-Abraham: Conceptualization; Supervision; Writing – review & editing.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethics and consent statement: This article conveys the opinions of the authors. Neither approval from an ethics committee nor informed consent is required.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Bahijja Tolulope Raimi-Abraham  https://orcid.org/0000-0002-5330-3967
https://orcid.org/0000-0002-5330-3967
References
- 1. LoBue P, Enarson D, Thoen C. Tuberculosis in humans and animals: an overview [Serialised article. Tuberculosis: a re-emerging disease in animals and humans. Number 1 in the series]. Int J Tuberc Lung Dis 2010; 14: 1075–1078. [PubMed] [Google Scholar]
- 2. World Health Organization. Global tuberculosis report. Geneva: World Health Organization, 2021. [Google Scholar]
- 3. Firdessa R, Tschopp R, Wubete A, et al. High prevalence of bovine tuberculosis in dairy cattle in central Ethiopia: implications for the dairy industry and public health. PLoS ONE 2012; 7(12): e52851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Awah-Ndukum J, Kudi A, Bah G, et al. Bovine tuberculosis in cattle in the highlands of Cameroon: seroprevalence estimates and rates of tuberculin skin test reactors at modified cut-offs. Vet Med Int 2012; 2012: 798502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu Y, Kang Q, Yang B, et al. Prevalence of bovine tuberculosis in the Aksu Region of Xinjiang, China, between 1985 and 2016. Arq Bras Med Vet Zoo 2019; 71: 374–378. [Google Scholar]
- 6. Teppawar RN, Chaudhari SP, Moon SL, et al. Zoonotic tuberculosis: a concern and strategies to combat. In: Enany S. (ed.) Basic biology and applications of actinobacteria. IntechOpen, 2018, https://www.intechopen.com/chapters/61347 [Google Scholar]
- 7. World Health Organization. Global TB report. Geneva: World Health Organization, 2020. [Google Scholar]
- 8. Cook RA, Karesh WB, Osofsky SA. The Manhattan Principles on ‘One world, One health’. In: One world, one health: building interdisciplinary bridges, New York, 29 September 2004. [Google Scholar]
- 9. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Geneva: World Health Organization, 2021. [Google Scholar]
- 11. World Health Organization. Guidelines on the management of latent tuberculosis infection. Geneva: World Health Organization, 2015. [PubMed] [Google Scholar]
- 12. Schraufnagel DE. ‘Latent tuberculosis infection’ is a term that should go dormant, and the significance of latent tuberculosis should be rethought. Ann Am Thorac Soc 2016; 13: 593–594. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization. World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization, 2019. [Google Scholar]
- 14. World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: World Health Organization, 2019. [PubMed] [Google Scholar]
- 15. World Health Organization. Definition of substandard and falsified (SF) medical products, https://www.who.int/news-room/fact-sheets/detail/substandard-and-falsified-medical-products (2017, accessed 10 January 2022).
- 16. World Health Organization. Substandard and falsified medical products, https://www.who.int/news-room/fact-sheets/detail/substandard-and-falsified-medical-products (2018, accessed 27 May 2021).
- 17. World Health Organization. A study on the public health and socioeconomic impact of substandard and falsified medical products. Geneva: World Health Organization, 2017. [Google Scholar]
- 18. Ogwal-Okeng JW, Owino E, Obua C. Chloroquine in the Ugandan market fails quality test: a pharmacovigilance study. Afr Health Sci 2003; 3(1): 2–6. [PMC free article] [PubMed] [Google Scholar]
- 19. Laferton JA, Kube T, Salzmann S, et al. Patients’ expectations regarding medical treatment: a critical review of concepts and their assessment. Front Psychol 2017; 8: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization. Antimicrobial resistance, https://www.who.int/en/news-room/fact-sheets/detail/antimicrobial-resistance (2020, accessed 27 May 2021).
- 21. Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health 2015; 109(7): 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Institute for Health and Care Excellence. Antimicrobial stewardship, https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/antimicrobial-prescribing-guidelines (2020, accessed 22 April 2022).
- 23. World Health Organization. What is DOTS? A guide to understanding the WHO-recommended TB control strategy known as DOTS. Geneva: World Health Organization, 1999. [Google Scholar]
- 24. World Health Organization. Global tuberculosis report. Geneva: World Health Organization, 2019. [Google Scholar]
- 25. World Health Organization. Guidelines for treatment of drug-susceptible tuberculosis and patient care. Geneva: World Health Organization, 2017. [Google Scholar]
- 26. Chang S, Cataldo JK. A systematic review of global cultural variations in knowledge, attitudes and health responses to tuberculosis stigma. Int J Tuberc Lung Dis 2014; 18(2): 168–173. [DOI] [PubMed] [Google Scholar]
- 27. Lara-Espinosa JV, Hernández-Pando R. Psychiatric problems in pulmonary tuberculosis: depression and anxiety. J Tuberc Res 2021; 9: 31. [Google Scholar]
- 28. Dye TD, Apondi R, Lugada ES, et al. ‘Before we used to get sick all the time’: perceptions of malaria and use of long-lasting insecticide-treated bed nets (LLINs) in a rural Kenyan community. Malaria J 2010; 9: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hutchins H, Power G, Ant T, et al. A survey of knowledge, attitudes and practices regarding malaria and bed nets on Bubaque Island, Guinea-Bissau. Malaria J 2020; 19: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Inah SA, Eko JE, Uwadiegwu Z, et al. Knowledge of malaria among adult residents in Abi local government area, cross River State, Nigeria. Asian J Res Med Pharm Sci 2017; 1: 1–9. [Google Scholar]
- 31. Tabong PT-N, Akweongo P, Adongo PB. Community beliefs about tuberculosis in Ghana: implications for the end tuberculosis global agenda. Cogent Med 2021; 8: 1870069. [Google Scholar]
- 32. Abebe G, Deribew A, Apers L, et al. Knowledge, health seeking behavior and perceived stigma towards tuberculosis among tuberculosis suspects in a rural community in southwest Ethiopia. PLoS ONE 2010; 5: e13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Getahun H, Aragaw D. Tuberculosis in rural northwest Ethiopia: community perspective. Ethiop Med J 2001; 39(4): 283–291. [PubMed] [Google Scholar]
- 34. Ngang P, Ntaganira J, Kalk A, et al. Perceptions and beliefs about cough and tuberculosis and implications for TB control in rural Rwanda. Int J Tuberc Lung Dis 2007; 11(10): 1108–1113. [PubMed] [Google Scholar]
- 35. Buregyeya E, Kulane A, Colebunders R, et al. Tuberculosis knowledge, attitudes and health-seeking behaviour in rural Uganda. Int J Tuberc Lung Dis 2011; 15(7): 938–942. [DOI] [PubMed] [Google Scholar]
- 36. Tobin EA, Okojie P-W, Isah EC. Community knowledge and attitude to pulmonary tuberculosis in rural Edo state, Nigeria. Ann Afr Med 2013; 12(3): 148–154. [DOI] [PubMed] [Google Scholar]
- 37. Viney KA, Johnson P, Tagaro M, et al. Tuberculosis patients’ knowledge and beliefs about tuberculosis: a mixed methods study from the Pacific Island nation of Vanuatu. BMC Public Health 2014; 14: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ayisi JG, van’t Hoog AH, Agaya JA, et al. Care seeking and attitudes towards treatment compliance by newly enrolled tuberculosis patients in the district treatment programme in rural western Kenya: a qualitative study. BMC Public Health 2011; 11: 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verhagen L, Kapinga R, van Rosmalen-Nooijens KA. Factors underlying diagnostic delay in tuberculosis patients in a rural area in Tanzania: a qualitative approach. Infection 2010; 38(6): 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walker I, Baral S, Wei X, et al. Multidrug-resistant tuberculosis treatment programmes insufficiently consider comorbid mental disorders. Int J Tuberc Lung Dis 2017; 21: 603–609. [DOI] [PubMed] [Google Scholar]
- 41. Alfawaz S, Alattas N, Alhammadi M, et al. Acute psychosis secondary to isoniazid in pediatric pulmonary tuberculosis: a case report and literature review. Int J Pediatr Adolesc Med 2020; 7(4): 196–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kanesalingavelan K, Sabhesan S, Savitha K, et al. Emotional disturbances among patients with pulmonary tuberculosis. Int J Contemp Med 2019; 7: 63–68. [Google Scholar]
- 43. Fantl K. Psychiatry and tuberculosis. Calif Med 1950; 73: 538–540. [PMC free article] [PubMed] [Google Scholar]
- 44. Van Rensburg AJ, Dube A, Curran R, et al. Comorbidities between tuberculosis and common mental disorders: a scoping review of epidemiological patterns and person-centred care interventions from low-to-middle income and BRICS countries. Infect Dis Poverty 2020; 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pachi A, Bratis D, Moussas G, et al. Psychiatric morbidity and other factors affecting treatment adherence in pulmonary tuberculosis patients. Tuberc Res Treat 2013; 2013: 489865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fatiregun A, Ejeckam C. Determinants of patient delay in seeking treatment among pulmonary tuberculosis cases in a government specialist hospital in Ibadan, Nigeria. Tanzan J Health Res 2010; 12: 113–120. [Google Scholar]
- 47. Zimmerman E, Smith J, Banay R, et al. Behavioural barriers and perceived trade-offs to care-seeking for tuberculosis in the Philippines. Glob Public Health. Epub ahead of print 4 December 2020. DOI: 10.1080/17441692.2020.1855460. [DOI] [PubMed] [Google Scholar]
- 48. Cremers AL, de Laat MM, Kapata N, et al. Assessing the consequences of stigma for tuberculosis patients in urban Zambia. PLoS ONE 2015; 10(3): e0119861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mitchison DA. How drug resistance emerges as a result of poor compliance during short course chemotherapy for tuberculosis [Counterpoint]. Int J Tuberc Lung Dis 1998; 2: 10–15. [PubMed] [Google Scholar]
- 50. Vernon A, Fielding K, Savic R, et al. The importance of adherence in tuberculosis treatment clinical trials and its relevance in explanatory and pragmatic trials. PLoS Med 2019; 16(12): e1002884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Basa S, Venkatesh S. Patient and healthcare system delays in the start of pulmonary tuberculosis treatment among tribal patients registered under DOTS, Odisha. J Clin Diagn Res 2016; 10(9): LC21–LC24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Asres M, Gedefaw M, Kahsay A, et al. Patients’ delay in seeking health care for tuberculosis diagnosis in East Gojjam zone, Northwest Ethiopia. Am J Trop Med Hyg 2017; 96(5): 1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lederberg J, Lederberg EM. Replica plating and indirect selection of bacterial mutants. J Bacteriol 1952; 63: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cairns J, Overbaugh J, Miller S. The origin of mutants. Nature 1988; 335: 142–145. [DOI] [PubMed] [Google Scholar]
- 55. Drake JW, Holland JJ. Mutation rates among RNA viruses. Proc Natl Acad Sci 1999; 96: 13910–13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen R, Quinones-Mateu ME, Mansky LM. Drug resistance, virus fitness and HIV-1 mutagenesis. Curr Pharm Des 2004; 10(32): 4065–4070. [DOI] [PubMed] [Google Scholar]
- 57. Woodford N, Ellington MJ. The emergence of antibiotic resistance by mutation. Clin Microbiol Infect 2007; 13: 5–18. [DOI] [PubMed] [Google Scholar]
- 58. Vogwill T, MacLean RC. The genetic basis of the fitness costs of antimicrobial resistance: a meta-analysis approach. Evol Appl 2015; 8(3): 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baquero F, Negri M. Strategies to minimize the development of antibiotic resistance. J Chemother 1997; 9: 29–37. [PubMed] [Google Scholar]
- 60. Pisani E. Antimicrobial resistance: what does medicine quality have to do with it. London: Review on Antimicrobial Resistance, 2015. [Google Scholar]
- 61. Seung KJ, Keshavjee S, Rich ML. Multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis. Cold Spring Harb Perspect Med 2015; 5: a017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Banerjee A, Dubnau E, Quemard A, et al. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 1994; 263: 227–230. [DOI] [PubMed] [Google Scholar]
- 63. Rouse DA, Li Z, Bai G-H, et al. Characterization of the katG and inhA genes of isoniazid-resistant clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother 1995; 39(11): 2472–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Taniguchi H, Aramaki H, Nikaido Y, et al. Rifampicin resistance and mutation of the rpoB gene in Mycobacterium tuberculosis. FEMS Microbiol Lett 1996; 144: 103–108. [DOI] [PubMed] [Google Scholar]
- 65. World Health Organization. Drug resistant TB, https://www.who.int/news/item/27-01-2021-who-announces-updated-definitions-of-extensively-drug-resistant-tuberculosis (2014, accessed 10 January 2022).
- 66. Aubry A, Pan X-S, Fisher LM, et al. Mycobacterium tuberculosis DNA gyrase: interaction with quinolones and correlation with antimycobacterial drug activity. Antimicrob Agents Chemother 2004; 48(4): 1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Escribano I, Rodriguez JC, Llorca B, et al. Importance of the efflux pump systems in the resistance of Mycobacterium tuberculosis to fluoroquinolones and linezolid. Chemotherapy 2007; 53(6): 397–401. [DOI] [PubMed] [Google Scholar]
- 68. Suzuki Y, Katsukawa C, Tamaru A, et al. Detection of kanamycin-resistant Mycobacterium tuberculosis by identifying mutations in the 16S rRNA gene. J Clin Microbiol 1998; 36(5): 1220–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ahmad K, Ahmad Z, Somayya R, et al. Analysis of rrs gene mutations in amikacin resistant clinical isolates of Mycobacterium tuberculosis from Khyber Pakhtunkhwa, Pakistan. Microb Pathog 2017; 108: 66–70. [DOI] [PubMed] [Google Scholar]
- 70. Maus CE, Plikaytis BB, Shinnick TM. Mutation of tlyA confers capreomycin resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 2005; 49(2): 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. van der Werf MJ, Langendam MW, Huitric E, et al. Multidrug resistance after inappropriate tuberculosis treatment: a meta-analysis. Eur Respir J 2012; 39: 1511–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Park JS, Lee J-Y, Lee YJ, et al. Serum levels of antituberculosis drugs and their effect on tuberculosis treatment outcome. Antimicrob Agents Chemother 2016; 60(1): 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bate R, Jensen P, Hess K, et al. Substandard and falsified anti-tuberculosis drugs: a preliminary field analysis. Int J Tuberc Lung Dis 2013; 17(3): 308–311. [DOI] [PubMed] [Google Scholar]
- 74. Berendes S, Heywood P, Oliver S, et al. Quality of private and public ambulatory health care in low and middle income countries: systematic review of comparative studies. PLoS Med 2011; 8(4): e1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lim M-K, Yang H, Zhang T, et al. Public perceptions of private health care in socialist China. Health Aff 2004; 23(6): 222–234. [DOI] [PubMed] [Google Scholar]
- 76. DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ 2003; 22(2): 151–185. [DOI] [PubMed] [Google Scholar]
- 77. DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ 2016; 47: 20–33. [DOI] [PubMed] [Google Scholar]
- 78. World Health Organization. Prioritisation of pathogens to guide discovery, research and development of new antibiotics for drug resistant bacterial infections including tuberculosis. Geneva: World Health Organization, 2017. [Google Scholar]
- 79. World Health Organization. World malaria report. Geneva: World Health Organization, 2019. [Google Scholar]
- 80. Kenyon T, Kenyon A, Kgarebe B, et al. Detection of substandard fixed-dose combination tuberculosis drugs using thin-layer chromatography. Int J Tuberc Lung Dis 1999; 3(11, Suppl. 3): S347–S350; discussion S351. [PubMed] [Google Scholar]
- 81. Zaki S, Bhongade S, Shanbag P. Red man syndrome due to accidental overdose of rifampicin. Indian J Crit Care Med 2013; 17(1): 55–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sridhar A, Sandeep Y, Krishnakishore C, et al. Fatal poisoning by isoniazid and rifampicin. Indian J Nephrol 2012; 22: 385–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ozawa S, Evans DR, Bessias S, et al. Prevalence and estimated economic burden of substandard and falsified medicines in low-and middle-income countries: a systematic review and meta-analysis. JAMA Netw Open 2018; 1: e181662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rahman MS, Yoshida N, Tsuboi H, et al. The health consequences of falsified medicines – A study of the published literature. Trop Med Int Health 2018; 23: 1294–1303. [DOI] [PubMed] [Google Scholar]
- 85. Trenchard H. Crisis in tuberculosis. Br Med J 1950; 2: 295. [Google Scholar]
- 86. Myers JA. Man’s greatest victory over tuberculosis. Baltimore, MD: C. C. Thomas, 1940. [Google Scholar]
- 87. Farmer PE. The consumption of the poor: tuberculosis in the 21st century. Ethnography 2000; 1: 183–216. [Google Scholar]
- 88. Shete PB, Reid M, Goosby E. Message to world leaders: we cannot end tuberculosis without addressing the social and economic burden of the disease. Lancet Glob Health 2018; 6(12): e1272–e1273. [DOI] [PubMed] [Google Scholar]
- 89. Geraldes Santos Mde L, Figueiredo Vendramini SH, Gazetta CE, et al. Poverty: socioeconomic characterization at tuberculosis. Rev Lat Am Enfermagem 2007; 15: 762–767. [DOI] [PubMed] [Google Scholar]
- 90. Gotham D, Fortunak J, Pozniak A, et al. Estimated generic prices for novel treatments for drug-resistant tuberculosis. J Antimicrob Chemother 2017; 72: 1243–1252. [DOI] [PubMed] [Google Scholar]
- 91. Ukwaja KN, Alobu I, Lgwenyi C, et al. The high cost of free tuberculosis services: patient and household costs associated with tuberculosis care in Ebonyi State, Nigeria. PLoS ONE 2013; 8(8): e73134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Aspler A, Menzies D, Oxlade O, et al. Cost of tuberculosis diagnosis and treatment from the patient perspective in Lusaka, Zambia. Int J Tuberc Lung Dis 2008; 12(8): 928–935. [PubMed] [Google Scholar]
- 93. Cherian JJ, Rahi M, Rinwa N, et al. India’s road to independence in manufacturing active pharmaceutical ingredients: focus on essential medicines. Economies 2021; 9: 71. [Google Scholar]
- 94. Quet M. Values in motion: anti-counterfeiting measures and the securitization of pharmaceutical flows. J Cult Econ 2017; 10: 150–162. [Google Scholar]
- 95. Zahedi M, Erfanmanesh M, Houshmand N. Anti-counterfeiting trade agreement and access to medicines in developing countries. Bioethics 2017; 7: 45–62. [Google Scholar]
- 96. Newton PN, Lee SJ, Goodman C, et al. Guidelines for field surveys of the quality of medicines: a proposal. PLoS Med 2009; 6: e1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. McManus D, Naughton BD. A systematic review of substandard, falsified, unlicensed and unregistered medicine sampling studies: a focus on context, prevalence, and quality. BMJ Glob Health 2020; 5(8): e002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tauqeer F, Myhr K, Gopinathan U. Institutional barriers and enablers to implementing and complying with internationally accepted quality standards in the local pharmaceutical industry of Pakistan: a qualitative study. Health Policy Plan 2019; 34: 440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. European Union. Directive 2011/62/EU of the European Parliament and of the Council of 8 June 2011 amending Directive 2001/83/EC on the Community code relating to medicinal products for human use, as regards the prevention of the entry into the legal supply chain of falsified medicinal products. Official J Eur Union. [Google Scholar]
- 100. Food and Drug Administration. Drug Supply Chain Security Act 2014. [Google Scholar]
- 101. Chatterjee B. Serialization and the Drug Quality & Security Act. Pharmaceutical Manufacturing, 20 January 2015, https://www.pharmamanufacturing.com/articles/2015/serialization-drug-quality-security-act/
- 102. Chircu AM, Sultanow E, Chircu FC. Cloud computing for big data entrepreneurship in the supply chain: using SAP HANA for pharmaceutical track-and-trace analytics. In: 2014 IEEE world congress on services, Anchorage, AK, 27 June–2 July 2014, pp. 450–451. New York: IEEE. [Google Scholar]
- 103. Nwankwo CA. THE ROLE OF NAFDAC IN COMBATING THE MARKETING OF SUB-STANDARD PHARMACEUTICAL DRUGS IN ANAMBRA STATE: A STUDY OF ONITSHA DRUG MARKET. Masters Thesis, Anambra State University, Igbariam, Nigeria, 2014. [Google Scholar]
- 104. Barton I, Avanceña ALV, Gounden N, et al. Unintended consequences and hidden obstacles in medicine access in Sub-Saharan Africa. Front Public Health 2019; 7: 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rasheed H, Höllein L, Holzgrabe U. Future information technology tools for fighting substandard and falsified medicines in low-and middle-income countries. Front Pharmacol 2018; 9: 995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Silva RBd, Mattos CAd. Critical success factors of a drug traceability system for creating value in a pharmaceutical supply chain (PSC). Int J Environ Res Public Health 2019; 16: 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhan J, Spennemann C. Ten actions to boost low and middle-income countries’ productive capacity for medicines, 2020, https://unctad.org/news/ten-actions-boost-low-and-middle-income-countries-productive-capacity-medicines
- 108. Parmaksiz K, Pisani E, Kok MO. What makes a national pharmaceutical track and trace system succeed? Lessons from Turkey. Glob Health: Sci Pract 2020; 8: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Foroudastan SD, Dees O. Solar power and sustainability in developing countries. In: Proceedings of the international conference on renewable energy for developing countries, 2006, pp. 1–13, https://files.udc.edu/docs/cere/Solar%20Power%20and%20Sustainability%20in%20Developing%20Countries.pdf
- 110. Sorato MM, Asl AA, Davari M. Implementation challenges of global end-to-end traceability system for pharmaceuticals in low and middle income countries: literature review. CPQ Med 2019; 7: 1–20. [Google Scholar]
- 111. Buckley GJ, Gostin LO. Countering the problem of falsified and substandard drugs. Washington, DC: National Academies Press, 2013. [PubMed] [Google Scholar]
- 112. Sproxil, https://sproxil.com (n.d., accessed 29 February 2020).
- 113. Dolan B. GlaxoSnithKline taps Sproxil for SMS drug authentication, https://www.mobihealthnews.com/10395/glaxosmithkline-taps-sproxil-for-sms-drug-authentication (2011, accessed 29 February 2020).
- 114. World Health Organization. Factors facilitating counterfeiting, https://apps.who.int/medicinedocs/en/d/Jh1456e/5.html (n.d., accessed 29 February 2020).
- 115. Pitkäranta A, Hayden FG. Trends in clinical practice rhinoviruses: important respiratory pathogens. Ann Med 1998; 30: 529–537. [DOI] [PubMed] [Google Scholar]
- 116. (IRACM) IIORACM. Problematic, https://www.iracm.com/en/fake-drugs/problematic/ (n.d., accessed 29 February 2020).
- 117. Binagwaho A, Bate R, Gasana M, et al. Combatting substandard and falsified medicines: a view from Rwanda. PLoS Med 2013; 10(7): e1001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Muvunyi CM, Ngabonziza JCS, Uwimana I, et al. Highly successful treatment outcome of multidrug-resistant and genetic diversity of multidrug-resistant Mycobacterium tuberculosis strains in Rwanda. Trop Med Int Health 2019; 24(7): 879–887. [DOI] [PubMed] [Google Scholar]
- 119. Government AUHoSa. AU model law on medical products regulation, 2016. https://www.nepad.org/nepad-oncontinent/african-medicines-regulatory-harmonisation-amrh-rwanda (2016, accessed 10 January 2022).