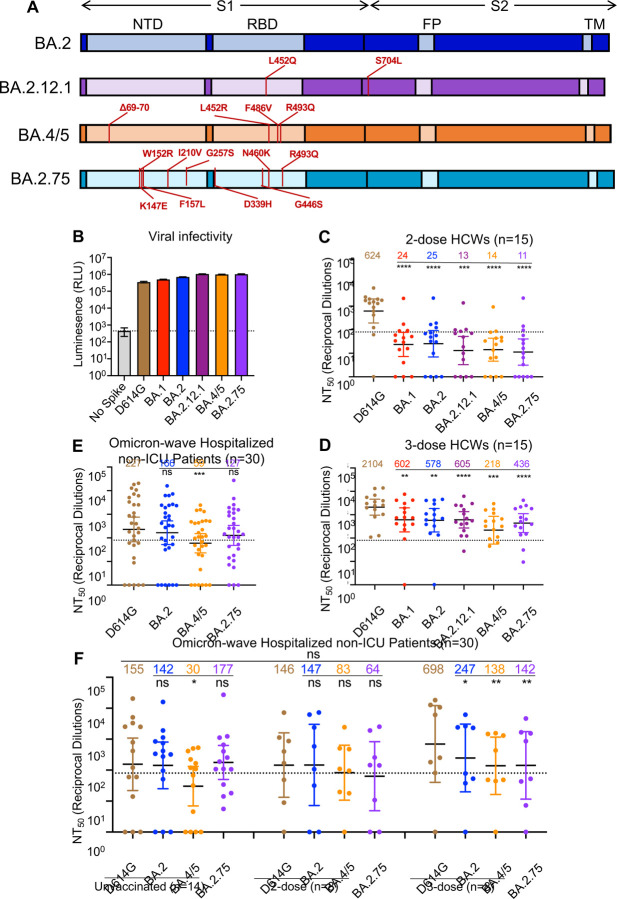
Figure 1: BA.2.75 exhibits strong neutralization resistance to 2-dose and 3-dose mRNA vaccinee sera and Omicron wave patient sera.
(A) Schematic of BA.2-derived SARS-CoV-2 variants with mutations relative to the BA.2 background indicated. Highlighted are the S1 and S2 subunits, N-terminal domain (NTD), receptor binding domain (RBD), fusion peptide (FP), and transmembrane domain (TM). (B) Infectivity of pseudotyped lentivirus bearing S protein from SARS-CoV-2 variants of study; bars represent means ± standard error. (C-D) Neutralizing antibody titers against lentivirus pseudotyped with S from individual SARS-CoV-2 variants for 15 health care workers for sera collected 3–4 weeks after second mRNA vaccination (C) or 1–12 weeks after homologous mRNA booster vaccination (D). (E) Neutralizing antibody titers for sera collected from 30 COVID-19 patients hospitalized during the BA.1 pandemic wave. (F) Neutralizing antibody titers against hospitalized BA.1 wave patients are divided by vaccination status. (C-F) Dots indicate individual patient samples; bars represent geometric means with 95% confidence intervals; significance relative to D614G was determined by one-way repeated measures ANOVA with Bonferroni multiplicity correction. P-values are displayed as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and ns for not significant.