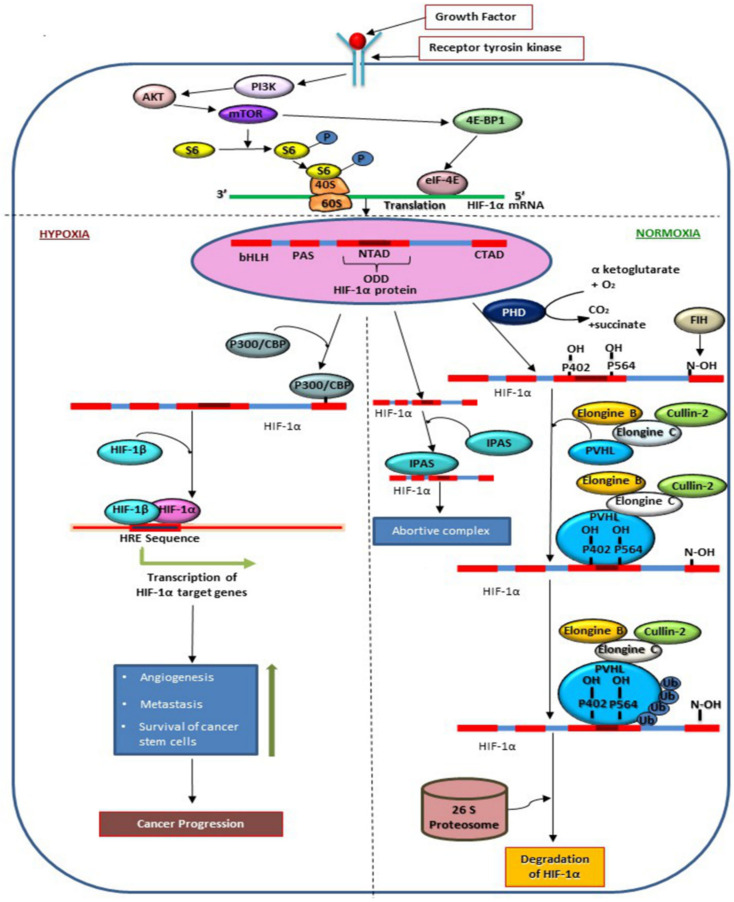
Figure 1.
Molecular mechanism of regulation of HIF-1α in hypoxic and normoxic conditions. The figure represents the induction of HIF-1α translation via PI3K/AKT/mTOR pathway. On binding to growth factors at receptor tyrosin kinase PI3K becomes activated which further induces AKT and mTOR pathway activation followed by phosphorylation of S6. HIF-1α synthesis is induced by eIF-4E which binds to HIF-1 α upon activation by 4E-BP1 which is a downstream signaling molecule of mTOR. In the presence of oxygen, Pro-402, Pro-564 in ODD and Asn-803 in CTAD are hydroxylated by PHD and FIH. As represented in the figure, hydroxylation at Asn-803 prevents binding of P300/CBP to HIF-1 α in normoxic conditions, whereas, hydroxylation at Proline residues allow VHL- elongine-C-elongine-B-Cullin-2 complex to bind at ODD of HIF-1α followed ubiquitination of HIF-1α via 26 proteasome. Expression of HIF-1α is also regulated by IPAS, a variant of HIF-3 which binds with HIF-1α to form an abortive complex. In hypoxic conditions, P300/CBP binds at CTAD which prevents degradation of HIF-1α. HIF-1α enters nucleus and forms active transcription factor by binding with HIF-1β in order to transcribe genes for angiogenesis, metastasis, and survival of cancer stem cells in tumor tissue.