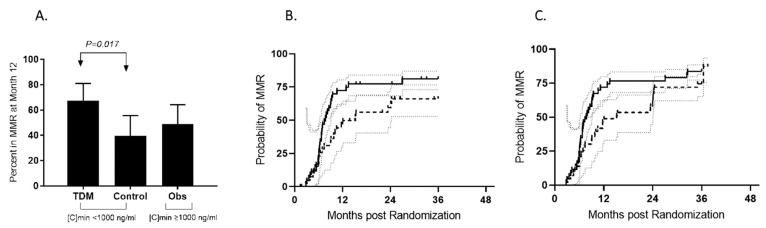
Figure 2.
Major molecular response rates at 12 months (A) and by 36 months (B,C). (A) Twenty-nine patients (67% (95% CI, 51–81)) achieved MMR at month 12 in the TDM arm, as opposed to 39% (95% CI, 24–55) in the control arm (p = 0.017) (dark plots). The rate of MMR was 49% (95% CI, 34–64) for patients included in the observational arm (dark plots). TDM: therapeutic drug monitoring arm, MMR: major molecular response (dark plots), no MMR: grey plots, [C)min: trough imatinib plasma level at inclusion. (B) Cumulative incidence of MMR in both the TDM arm (continuous line) and the control arm (dashed line) during the 36-month study period. Patients were censored in case of imatinib cessation (as is usual for studies comparing imatinib and second-generation tyrosine kinase inhibitors [6,7,8]). (C) The cumulative incidence of MMR in both the TDM arm (continuous line) and the control arm (dashed line) during the 36-month study period. Patients were not censored in case of imatinib cessation.