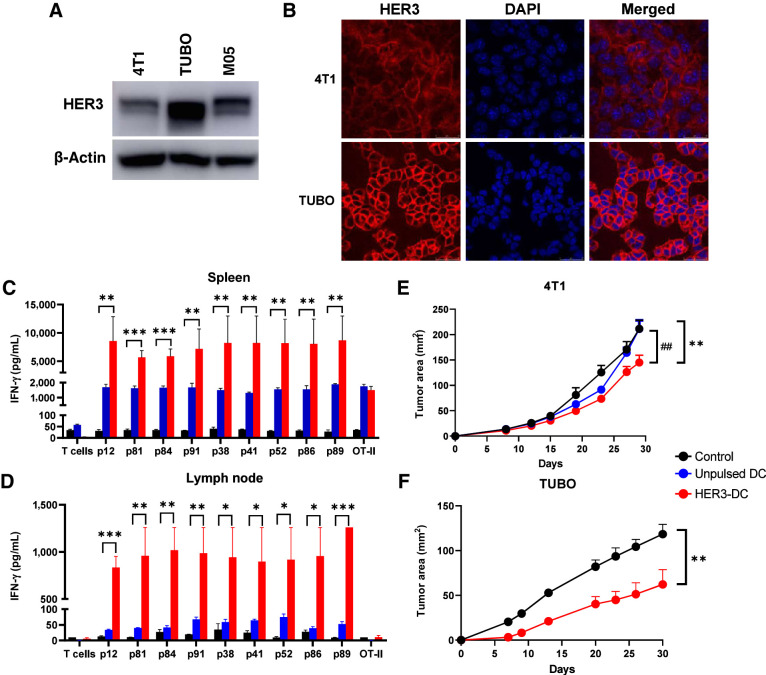
Figure 3.
HER3-DC1 vaccination elicits peptide-specific immune response and delays tumor growth. A, Immunoblotting of murine tumor cell lines 4T1, TUBO, and M05 to detect HER3. β-Actin: loading control. B, Immunofluorescence for HER3 (red) and nucleus (DAPI, blue) in 4T1 and TUBO murine mammary tumor cells (image magnification: 1,200×). C and D, Individual HER3 peptide–specific immune responses in spleens (C) and lymph node–derived immune cells (D) from control (black), unpulsed mature DC1 (blue), and HER3-DC1 (red) vaccinated BALB/c mice (n = 3). Spleens were processed, and splenocytes were restimulated with the HER3 peptides for 72 hours to detect IFN-γ by ELISA. Lymph node–derived lymphocytes were cocultured with DC1 pulsed with individual HER3 peptides for 72 hours to detect IFN-γ by ELISA. E, Tumor growth after 4T1 tumor challenge in control (black), unpulsed mature DC1 (blue), and HER3-DC1 (red) vaccinated mice (n = 7–10 mice/group). Mice were challenged 2 weeks after the last vaccination and were monitored until endpoint. *, control versus HER3-DC1; #, unpulsed DC1 versus HER3-DC1. F, TUBO tumor growth in control (black) and HER3-DC1 (red) vaccinated mice (n = 7–10 mice/group). Mice were challenged 2 weeks after the last vaccination. Data represented as mean ± SEM with statistical significance determined using multiple t test without correction for multiple comparisons. Each row analyzed individually, without assuming a consistent SD. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ##, P ≤ 0.01.