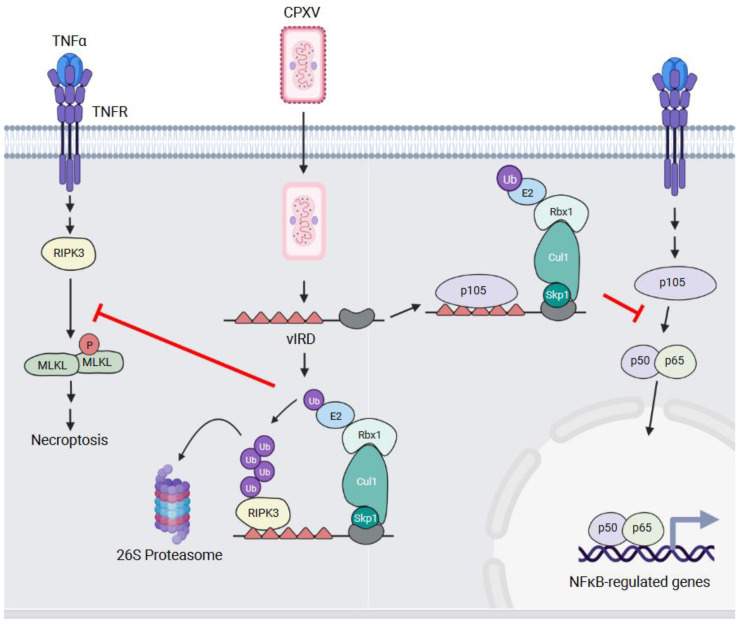
Figure 5.
vIRD—a regulator of necroptotic and inflammatory signalling. TNFα binding to the TNFR initiates signalling events that lead to the activation of the RIPK3 serine/threonine kinase. RIPK3 phosphorylates the MLKL protein which results in MLKL multimerization, membrane permeabilization, and the induction of necroptosis. In CPXV-infected cells, vIRD blocks necroptosis by preventing MLKL phosphorylation through binding, ubiquitylating, and targeting RIPK3 to the proteasome for degradation. vIRD also binds the p105 NFKB1 protein, but it does not promote p105 NFKB1 degradation. Rather, this interaction appears to block the processing of p105 NFKB1 into the p50 subunit. This prevents p50-containing NF-κB dimers from translocating to the nucleus and promoting the transcription of NF-κB-regulated genes in response to TNFR signalling.