Abstract
Background
The mortality rate for a patient with a refractory cardiogenic shock on venoarterial (VA) extracorporeal membrane oxygenation (ECMO) remains high, and hyperoxia might worsen this prognosis. The objective of the present study was to evaluate the association between hyperoxia and 28-day mortality in this setting.
Methods
We conducted a retrospective bicenter study in two French academic centers. The study population comprised adult patients admitted for refractory cardiogenic shock. The following arterial partial pressure of oxygen (PaO2) variables were recorded for 48 h following admission: the absolute peak PaO2 (the single highest value measured during the 48 h), the mean daily peak PaO2 (the mean of each day’s peak values), the overall mean PaO2 (the mean of all values over 48 h), and the severity of hyperoxia (mild: PaO2 < 200 mmHg, moderate: PaO2 = 200–299 mmHg, severe: PaO2 ≥ 300 mmHg). The main outcome was the 28-day all-cause mortality. Inverse probability weighting (IPW) derived from propensity scores was used to reduce imbalances in baseline characteristics.
Results
From January 2013 to January 2020, 430 patients were included and assessed. The 28-day mortality rate was 43%. The mean daily peak, absolute peak, and overall mean PaO2 values were significantly higher in non-survivors than in survivors. In a multivariate logistic regression analysis, the mean daily peak PaO2, absolute peak PaO2, and overall mean PaO2 were independent predictors of 28-day mortality (adjusted odds ratio [95% confidence interval per 10 mmHg increment: 2.65 [1.79–6.07], 2.36 [1.67–4.82], and 2.85 [1.12–7.37], respectively). After IPW, high level of oxygen remained significantly associated with 28-day mortality (OR = 1.41 [1.01–2.08]; P = 0.041).
Conclusions
High oxygen levels were associated with 28-day mortality in patients on VA-ECMO support for refractory cardiogenic shock. Our results confirm the need for large randomized controlled trials on this topic.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04133-7.
Keywords: Cardiogenic shock, ECMO, Hyperoxia, Mortality
Background
Venoarterial extracorporeal membrane oxygenation (VA-ECMO) is recommended as a rescue therapy for ensuring organ perfusion and oxygen delivery in cases of refractory cardiogenic shock (CS) [1]. The survival rate for CS patients on VA-ECMO varies from one study to another; the largest study to date (an analysis of more than 2000 patients documented in the Extracorporeal Life Support Organization database) found a value of 40% [2].
A large number of factors can influence the mortality of CS patients on VA-ECMO support, including patient characteristics, the etiology of the CS, the center’s level of experience, and adverse bleeding/thrombotic events [3–6]
Hyperoxia is a modifiable factor that reportedly worsens outcomes in several critical illnesses and might contribute to the elevated mortality rates observed in patients with refractory CS [7]. The hypothesis is that hyperoxia might increase oxidative stress by triggering enzymatic pathways that result in greater production of free radicals and reactive oxygen species (ROS) [8]. This elevation in ROS generation might promote neutrophil activation, which might in turn lead to an inappropriate inflammatory response [9], 10. Lung injury associated with hyperoxia is well described with loss in hypoxic pulmonary vasoconstriction and formation of atelectasis [11]. Therapeutics issues from animal models are described to protect the lung against hyperoxia injuries as the use of luciferase acting like an antioxidant reduces the production of ROS [12]. However, clinical reports on oxygen management during VA-ECMO support for CS are scarce, and the data on hyperoxia in this setting are contradictory. An analysis of the Extracorporeal Life Support Organization database did not find an association between hyperoxia and VA-ECMO [13]. However, the analysis included patients with refractory CS and those with refractory cardiac arrest, which means that the data must be interpreted with caution [13], 14. Although hyperoxia appears to be associated with mortality during venovenous ECMO [13], the data on VA-ECMO in CS are inconsistent [15].
We hypothesized that in a homogenous population of patients with low cardiac output and severe organ oxygen deprivation, hyperoxia might compromise the function of organs with ischemia perfusion injuries during initial patient management with VA-ECMO.
The objective of the study was therefore to evaluate the putative association between early hyperoxia and 28-day mortality in patients on VA-ECMO support.
Methods
Setting and ethics
This retrospective, multicenter study was performed in three intensive care units (ICUs) at two university medical centers (Unité de Réanimation Cardio-Vasculaire, Institut Cœur-Poumon, Lille University Medical Center, Lille, France; Pôle des Réanimations, Hôpital Salengro, Lille University Medical Center, Lille, France; Unité de Réanimation Cardio-Thoracique, Vasculaire et Respiratoire, Amiens University Medical Center, Amiens, France). The study was approved by the investigational review board at the French Society of Anesthesia, Intensive Care and Perioperative Medicine (Paris, France) on January 17, 2022 (reference: CERAR IRB 00,010,254-2022-009). In accordance with French legislation, all datasets used in the present study were registered with the French National Data Protection Commission (Commission nationale de l'informatique et des libertés (Paris, France; methodology MR-004; reference: 2208336v0 for Amiens University Medical Center, and DEC2015-14 for Lille University Medical Center) [16].
Study population
Consecutive adult patients on VA-ECMO support for refractory CS rated as Interagency Registry for Mechanically Assisted Circulatory Support profile 1 or 2 (“crash and burn” and “progressive decline on inotropic support,” respectively) or Society for Cardiovascular Angiography and Interventions stage D or E (“deteriorating” and “extremis,” respectfully) between January 2013 until January 2020 were considered for inclusion [17, 18].
ECMO initiated exclusively for high-risk percutaneous coronary intervention, cardiopulmonary resuscitation, or an intraoperative procedure was not included in the study. The formal exclusion criteria were age under 18, a moribund patient (death within 48 h of initiating VA-ECMO), missing data for PaO2, uncertainty as to whether a blood sample came from the right arterial or ulnar arteries (in cases of femoral artery cannulation), a second ECMO run in the same patient, central cannulation, and right ventricle to pulmonary artery ECMO.
Data collection
Clinical data and outcome data were gathered from paper-based or electronic medical records [Sillage (SIB, Rennes, France) and IntelliSpace Critical Care and Anesthesia (Philips Healthcare, Koninklijke Philips N.V., the Netherlands) for the Lille centers and Centricity Critical Care (formerly known as Clinisoft) software (GE Healthcare, Barrington, IL) for the Amiens center. Laboratory information was collected from devoted software applications [Molis® (CompuGroup Medical, Koblenz, Germany) in the Lille centers and Clinisoft (GE Healthcare, Barrington, IL) in the Amiens center]. We collected anthropometric data, a detailed medical history, the Simplified Acute Physiologic Score (SAPS II), the baseline arterial blood lactate level, and other laboratory variables. We also collected all relevant information concerning ECMO management, including the indication for ECMO support, the cannulation site, the duration of cannulation, the type of device, the ECMO flow, and the occurrence of any ECMO-related complications.
Blood samples and laboratory analysis
Arterial blood samples were drawn into a 3-mL preheparinized syringe (BD Preset™, Plymouth, UK) from arterial lines positioned in the right radial artery. The samples were processed within minutes of collection on a point-of-care analyzer or sent to a central laboratory using an automatized pneumatic tube transportation system that shortened the laboratory delivery time to a few minutes. PaO2 was measured using an ABL90 FLEX or ABL800 FLEX blood gas analyzer (Radiometer® Medical ApS, Brønshøj, Denmark) or a GEM®Premier 4000 blood gas analyzer (Instrumentation Laboratory, Werfen, Bedford, MA, USA) depending on the center.
Management of VA-ECMO
The femoral artery or the right subclavian artery was cannulated percutaneously or with a semi-Seldinger approach. The femoral vein was cannulated by trained cardiovascular or thoracic surgeons. During the study period, three ECMO systems were in use: a Maquet ECMO system, comprising a Rotaflow centrifugal pump-based system and a Cardiohelp with a disposable 5.0/7.0HLS Set Advanced (Getinge AB, Göteborg, Sweden); a LivaNova system (LivaNova, Saluggia, Italy) with a revolution pump head; and a Eurosets system (Eurosets Srl, Medolla, Italy)].
Anticoagulation was initiated with a 100 IU/kg bolus of unfractionated heparin before cannulation for patients not on cardiopulmonary bypass prior to ECMO support, followed by a continuous infusion. The target was an anti-FXa level of 0.2–0.4 IU ml−1 in the Lille and Amiens centers.
The pump flow was adjusted to target a mean arterial pressure > 60 mmHg, SvO2 > 65% or ScVO2 > 70%, and aortic valve opening. Weaning was considered for recovery when the cardiac output was acceptable after reducing the ECMO flow and inotropic support to the minimum level.
It should be noted that PaO2 was adjusted at the discretion of the attending physician—primarily by changing the ECMO system’s fraction of inspired oxygen (FiO2) via an oxygen–air blender (Sechrist Industries, Anaheim, CA). The FiO2 on the ventilator was set to the minimum value and was modified as a function of the arterial oxygen saturation (SaO2) measured in the right upper limb arteries (for femoral cannulation) or the left upper limb arteries (for right subclavian arterial cannulation) or the cerebral near-infrared spectrometry index values when the SaO2 or peripheral oxygen saturation (SpO2) values were unavailable.
Definition of oxygen parameters
The following PaO2 values (in mmHg) were recorded or calculated over the first 48 h following admission to the ICU: (i) the three through PaO2 values on admission (Day 0), Day 1, and Day 2; (ii) the three peak PaO2 values on admission, Day 1, and Day 2; (iii) the mean daily peak PaO2 (mmHg), calculated as the mean of the daily peak values on admission, Day 1, and Day 2; (iv) the mean daily through PaO2, calculated as the mean of the daily through values; (v) the absolute peak PaO2, i.e., the highest peak PaO2 value between admission and Day 2; (vi) the overall mean PaO2, i.e., the mean of all PaO2 values between admission and Day 2; and (vi) the severity of hyperoxia, graded with reference to the overall mean PaO2 (mild: < 200 mmHg; moderate: 200–299 mmHg; severe: ≥ 300 mmHg).
Study endpoint
The primary study endpoint was 28-day all-cause mortality, as determined from the patient’s electronic medical records and the French national death registry (Institut national des statistiques et des études économiques, Paris, France) [19]. None of the study participants was lost to follow-up, and status at 28 days could be documented in all cases.
Statistical analysis
Data were presented as the mean ± standard deviation or the frequency (percentage), as appropriate. Non-survivors were compared with 28-day survivors using Student’s t test, a chi-squared test, or Fisher’s exact test, as appropriate. To assess the effects of ECMO flow rate and hemoglobin on PaO2, we used a multiple linear regression. Binary logistic regression analyses with 28-day mortality as the dependent variable were used to estimate the respective univariate associations with the absolute peak PaO2, mean daily peak PaO2, the overall mean PaO2, and the hyperoxia range. Unadjusted and adjusted odds ratios (ORs) with their 95% confidence interval (CI) were estimated from the binary logistic regression for a 10-point increment in PaO2-derived parameters. The results were adjusted for age, hypertension, the indication for VA-ECMO support, the SAPS II, and the arterial blood lactate on admission. Kaplan–Meier estimates were used for time-to-event analyses. Sensitivity analyses were performed by excluding patients with femoro-axillary ECMO and by using the OR as a measure of the effect size.
Missing data have been imputed for the following stages using predictive mean matching imputation (pmm). Five imputation data sets were produced. Missing data have been analyzed as missing-not-at-random according to the Rubin rule [20]. Prognostic variables related to 28-day mortality at the 20% in univariate analysis were included in the propensity score (regardless of their differences between the two groups): age, gender, hypertension, eGFR, diabetes, coronary disease, SAPS II, arterial lactate on admission, and etiology of refractory cardiogenic shock. For each patient, the probability to show hyperoxia has been estimated using logistic regression. For ATE (average treatment effect on the entire population) analysis, weights have been attributed to each patient of the hyperoxia (overall mean PaO2 > 150 mmHg) and no hyperoxia (overall mean PaO2 ≤ 150 mmHg) groups, making the two groups similar for the variables in the propensity score [21]. These weights were calculated using the stabilized inverse probability of treatment weighting (SIPTW). Balance (standardized mean differences lower than 10%) was checked for each variable. ORs were estimated before and after weighting on the propensity score using logistic regression. All tests were two-sided, and the threshold for statistical significance was set to p < 0.05. Statistical analysis was performed with R studio software for macOS (version 2021.09.1 + 372) and its «dplyr», «ggplot2», «survminer», «survival», « hrbrthemes», «tableone», « ggeffects», « WeightIt», « cobalt», « compareGroups», « mice» and « epiR» and “reshape2” packages.
Results
Study population
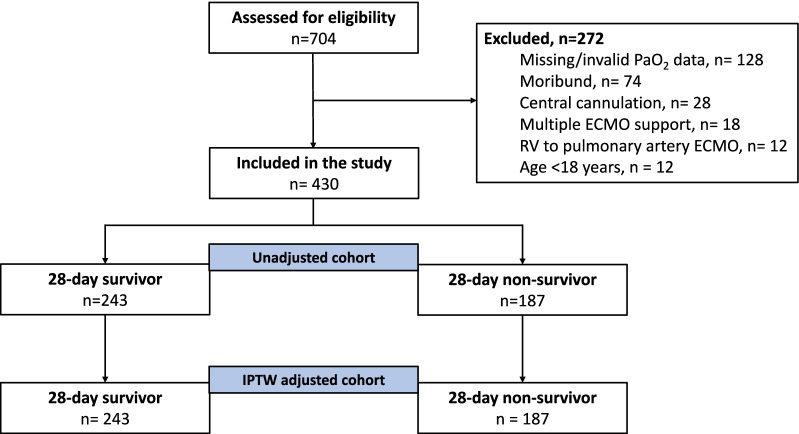
From January 2013 to January 2020, a total of 704 patients received VA-ECMO support; 272 did not meet the inclusion criteria, and so the final analysis comprised 430 patients (Fig. 1).
Fig. 1.
Flowchart. ECMO: extracorporeal membrane oxygenation; RV: right ventricle; IPTW: inverse probability of treatment weighting
Baseline characteristics
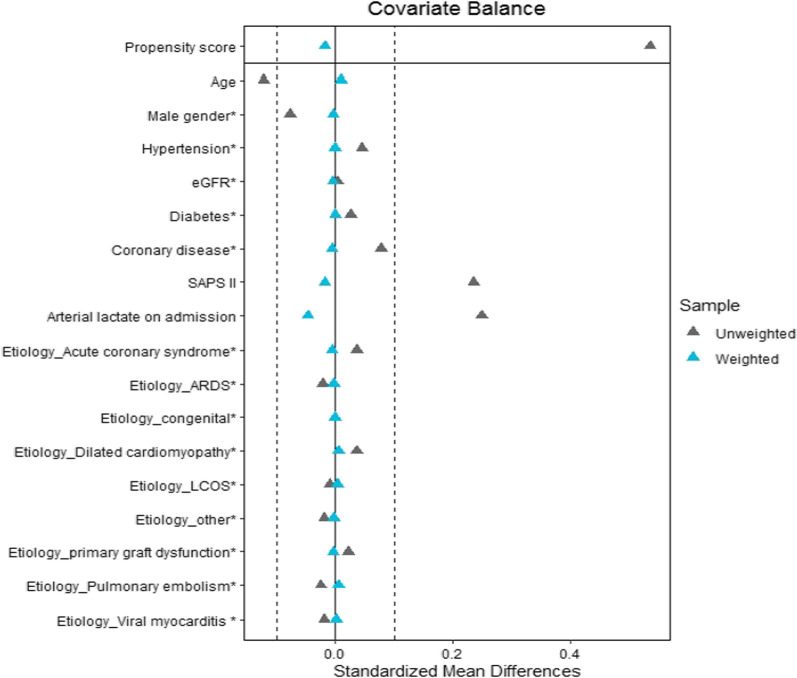
Relative to survivors, non-survivors were significantly older and more likely to have a history of hypertension (Table 1). The non-survivors had a significantly greater SAPS II and arterial blood lactate level on admission. Before weighting, the prevalence of acute coronary syndrome and low cardiac output syndrome was significantly higher in non-survivors. The 28-day mortality rate was 43%. After weighting, the mean standard differences were inferior to 15% for all baseline characteristics as presented in Table 1 and in the love plot (Fig. 2).
Table 1.
Baseline characteristics of patients (28-day survivors vs. non-survivors) on venoarterial ECMO support before and after weighting
| Before weighting | After weighting | |||||
|---|---|---|---|---|---|---|
| Variable | Survivors (n = 243) |
Non-survivors (n = 187) |
SMD | Survivors (n = 243) |
Non-survivors (n = 187) |
SMD |
| Age; years | 53 ± 14 | 59 ± 13 | 0.122 | 58 ± 10 | 58 ± 10 | 0.010 |
| Male sex; n (%) | 155 (64) | 134 (72) | 0.165 | 168 (69) | 127 (68) | 0.008 |
| BMI | 27.6 ± 6.1 | 28.0 ± 5.8 | 0.084 | 27.9 ± 6 | 27.9 ± 7 | 0.046 |
| Medical history; n (%) | ||||||
| Hypertension | 102 (42) | 102 (55) | 0.090 | 119 (49) | 92 (49) | 0.001 |
| eGFR < 80 ml min−1 m−2 | 103 (43) | 98 (53) | 0.007 | 116 (48) | 90 (49) | 0.008 |
| Diabetes | 54 (22) | 54 (29) | 0.059 | 61 (25) | 47 (25) | < 0.001 |
| Stroke | 14 (6) | 13 (7) | 0.009 | 17 (7) | 13 (7) | 0.003 |
| Coronary disease | 99 (41) | 90 (49) | 0.156 | 109 (45) | 84 (45) | 0.011 |
| COPD/asthma | 22 (9) | 23 (12) | 0.132 | 27 (11) | 23 (12) | 0.109 |
| Dilated cardiomyopathy | 31 (13) | 19 (10) | 0.09 | 29 (12) | 21 (11) | 0.04 |
| Valvular heart disease | 50 (21) | 46 (25) | 0.173 | 56 (23) | 43 (23) | 0.149 |
| SAPS II | 54 [38–71] | 57 [45–74] | 0.235 | 56 [43–73] | 54 [43–72] | 0.018 |
| Arterial lactate on admission; mmol l−1 | 6 ± 4 | 8 ± 5 | 0.249 | 5 ± 4 | 5 ± 4 | 0.044 |
| Etiology of refractory cardiogenic shock; n (%) | ||||||
| LCOS | 63 (26) | 54 (29) | 0.265 | 66 (27) | 51 (27) | 0.038 |
| Primary graft dysfunction | 16 (7) | 4 (2) | 12 (5) | 9 (5) | ||
| Acute coronary syndrome | 63 (26) | 58 (31) | 68 (28) | 52 (28) | ||
| Dilated cardiomyopathy | 37 (15) | 16 (9) | 27 (11) | 22 (12) | ||
| Viral myocarditis | 13 (5) | 4 (2) | 10 (4) | 7 (4) | ||
| Pulmonary embolism | 13 (5) | 9 (5) | 12 (5) | 11 (6) | ||
| Congenital | 3 (1) | 1 (1) | 3 (1) | 2 (1) | ||
| ARDS | 5 (2) | 5 (3) | 5 (2) | 4 (2) | ||
| Other | 30 (12) | 30 (20) | 39 (16) | 30(16) | ||
BMI, body mass index; eGFR, estimated glomerular filtration rate; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; SAPS II, Simplified Acute Physiology Score II; LCOS, low cardiac output syndrome; ARDS, acute respiratory distress syndrome; SMD: standardized mean differences
Fig. 2.
Love plots for standardized mean differences comparing covariate values before (gray triangle) and after (blue triangle) propensity score weighting for the assessment of 28-day mortality. LCOS: low cardiac output syndrome; SAPS II: Simplified Acute Physiology Score II; eGFR: estimated glomerular filtration rate; ARDS: acute respiratory distress syndrome
Comparisons of oxygen levels in survivors versus non-survivors
Survivors and non-survivors did not differ significantly with regard to the daily through PaO2 from ICU admission to Day 2 (Table 2). The daily peak PaO2 was significantly higher in non-survivors than in survivors on admission (mean ± SD: 258 ± 111 vs. 228 ± 100 mmHg, respectively; P = 0.007) and on Day 1 (and 237 ± 93 vs. 208 ± 87 mmHg, respectively; P = 0.001) but not on Day 2. The non-survivors and survivors did not differ significantly with regard to the daily through PaO2 (94 ± 44 vs. 92 ± 37 mmHg, respectively; P = 0.567). The mean daily peak PaO2, the absolute peak PaO2, and the overall mean PaO2 were significantly higher in non-survivors than in survivors (respectively 221 ± 66 vs. 202 ± 51 mmHg; P = 0.004, 296 ± 100 vs. 265 ± 90 mmHg; P = 0.002 and 158 ± 42 vs. 147 ± 39 mmHg; P = 0.011). The prevalence of moderate and severe hyperoxia was significantly higher in non-survivors than in survivors.
Table 2.
Daily peak, daily through, and mean PaO2 values in 28-day survivors and non-survivors
| Variables | Survivors (n = 243) | Non-survivors (n = 187) | P value |
|---|---|---|---|
| Inspired oxygen fraction (%) | |||
| ICU admission | 0.5 ± 0.1 | 0.7 ± 0.2 | < 0.001 |
| Day 1 | 0.5 ± 0.1 | 0.7 ± 0.2 | < 0.001 |
| Day 2 | 0.5 ± 0.1 | 0.6 ± 0.2 | < 0.001 |
| Daily through PaO2; mmHg | |||
| ICU admission | 108 ± 68 | 109 ± 85 | 0.844 |
| Day 1 | 84 ± 37 | 86 ± 48 | |
| Day 2 | 86 ± 34 | 94 ± 49 | 0.053 |
| Daily peak PaO2; mmHg | |||
| ICU admission | 228 ± 100 | 258 ± 111 | 0.007 |
| Day 1 | 208 ± 87 | 237 ± 93 | 0.001 |
| Day 2 | 183 ± 74 | 191 ± 83 | 0.369 |
| Mean daily peak PaO2; mmHg a | 202 ± 61 | 221 ± 66 | 0.004 |
| Mean daily through PaO2; mmHg b | 92 ± 37 | 94 ± 44 | 0.567 |
| Absolute peak PaO2 over 48 h; mmHg | 265 ± 90 | 296 ± 100 | 0.002 |
| PaO2 range; n (%) | |||
| < 200 mmHg | 152 (63) | 30 (40) | 0.010 |
| 200 – 299 mmHg | 74 (30) | 76 (41) | |
| ≥ 300 mmHg | 17 (7) | 19 (10) | |
| Overall mean PaO2; mmHg c | 147 ± 39 | 158 ± 42 | 0.011 |
a:The mean daily peak PaO2 is the mean of the three daily peak PaO2 values (measured on admission (Day 0), Day 1, and Day 2). b: The mean daily through PaO2 is the mean of the three of the daily through PaO2 values (measured on admission, Day 1, and Day 2). c: The overall mean PaO2 is the mean of all PaO2 values measured between admission and Day 2. ICU: intensive care unit
Influence of ECMO flow rate and hemoglobin on PaO2 level at admission
In a multiple regression model, hemoglobin was significantly associated with PaO2 with an estimated regression coefficient at − 10.5 g dl−1 (95% CI − 16.3 to − 4.8) per 1 mmHg increase in PaO2 (P < 0.001). ECMO flow rate was not associated with PaO2 at admission with an estimated regression coefficient at 4.5 l min−1 (95% CI − 9.1 to 18.2) per 1 mmHg increase in PaO2 (P = 0.51). The association between hemoglobin, ECMO flow rate, and PaO2 is presented in Additional file 1: Fig. S1.
Univariate and multivariate analyses
In a univariate logistic regression, the mean daily peak PaO2, the absolute peak PaO2, the overall mean PaO2, and the hyperoxia range were significantly associated with 28-day mortality (Table 3). After adjustment for age, hypertension, the indication for VA-ECMO, the SAPS II, and the arterial blood lactate on admission, we found that the mean daily peak PaO2, the absolute peak PaO2, the overall mean PaO2, and the hyperoxia range were independent predictors of 28-day mortality (OR [95%CI] per 10 mmHg increment: 2.65 [1.79–6.07], P = 0.02; 2.36 [1.67–4.82], P = 0.018; 2.85 [1.12–7.37], P = 0.028, respectively). Moderate and severe hyperoxia were found to be independent predictors of 28-day mortality. The predicted probability of 28-day mortality according to the mean daily peak PaO2, the absolute peak PaO2, and the overall mean PaO2 is presented in Additional file 2: Fig. S2.
Table 3.
Association between hyperoxia and 28-day mortality, before and after adjustment
| Variables | Unadjusted OR [95%CI] for a 10 mmHg increment | P value | Adjusteda OR [95%CI] for a 10 mmHg increment | P value |
|---|---|---|---|---|
| Mean daily peak PaO2; mmHgb | 2.77 [1.38–5.07] | 0.005 | 2.65 [1.79–6.07] | 0.02 |
| Absolute peak PaO2; mmHg | 2.48 [1.35–4.62] | 0.004 | 2.36 [1.67–4.82] | 0.018 |
| PaO2 range; n (%) | ||||
| 200 mmHg | 1 (reference) | – | 1 (reference) | – |
| 200–299 mmHg | 1.88 [1.21–2.96] | 0.005 | 1.82 [1.10–3.00] | 0.02 |
| ≥ 300 mmHg | 2.06 [1.00–4.27] | 0.05 | 2.20 [1.00–5.31] | 0.002 |
| Overall mean PaO2; mmHg c | 2.66 [1.22–5.96] | 0.015 | 2.85 [1.12–7.37] | 0.028 |
OR: odds ratio. a: ORs were obtained from a multivariate logistic regression with adjustment for age, hypertension, the indication for extracorporeal life support, the arterial blood lactate level on admission, and the Simplified Acute Physiology Score II. The ORs for the mean daily peak PaO2, absolute peak PaO2, and the mean PaO2 over 48 h were calculated for a 10-point increment in PaO2. b: The mean daily peak PaO2 is the mean of the three daily peak PaO2 values (measured on admission (Day 0), Day 1, and Day 2). c: The overall mean PaO2 is the mean of all PaO2 values measured between admission and Day 2
Association between high level of oxygen and 28-day mortality
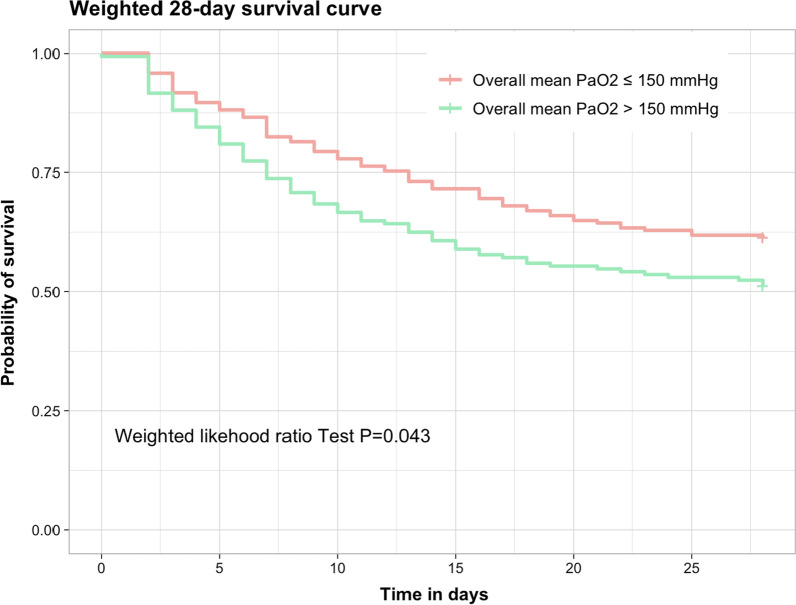
Before weighting, a high level of oxygen (overall mean PaO2 > 150 mmHg) was significantly associated with 28-day mortality with an OR at 1.51 [1.03–2.23] (P = 0.035). After weighting, the association remained significantly different with an OR at 1.41 [1.01–2.08] (P = 0.041). Time to 28-day survival was significantly better in patients with an overall mean PaO2 ≤ 150 mmHg (Fig. 3).
Fig. 3.
Propensity weighted Kaplan–Meier 28-day survival curve according to the overall mean PaO2 within the first 48 h after admission
Survival analysis, by hyperoxia range
In a Kaplan–Meier analysis, mild hyperoxia was associated with greater 28-day survival, relative to moderate and severe hyperoxia (log-rank test: P = 0.0052) (Additional file 3: Fig. S3).
Sensitivity analysis
Sixty-one patients had received femoro-axillary ECMO support. After the exclusion of these patients from the analysis, the OR for the association between oxygen parameters and 28-day mortality remained broadly consistent with the primary results (Additional file 4: Table S1).
Discussion
Our findings suggest that exposure to hyperoxia during the early management of refractory CS is associated with a greater 28-day mortality rate. We also demonstrate a dose effect of the PaO2 increase on the risk of 28-day mortality, whatever the oxygen parameter assessed.
As mentioned above, published data on hyperoxia in patients on VA-ECMO are scarce. Hyperoxia seems frequent in the early management of VA-ECMO with almost 1 out of 2 patients concerned by the level of PaO2 over 300 mmHg [22]. However, the impact on outcomes is uncertain regarding the results of the two main studies with some contradictions: Al Kawaz et al. found a significant association between hyperoxia and mortality, whereas Munshi et al. did not [15], [13]. This disparity might have been due to differences in the study participants’ blood oxygen levels. Indeed, PaO2 was around 200 mmHg in Al Kawaz et al.’s study but only around 100 mmHg in Munshi et al.’s study. Our values were close to those reported by Al Kawaz et al.—confirming the risk of mortality at ~ 200 mmHg and over. Heterogeneity in the study population might be another explanatory factor. Munshi et al.’s study population was heterogeneous (including patients on venovenous ECMO or extracorporeal cardiopulmonary resuscitation, as well as patients on VA-ECMO for refractory CS (situations with marked differences in the mortality rate and pathophysiology), whereas Al Kawaz et al. focused on patients receiving VA-ECMO.
The duration of exposure to hyperoxia is probably also an important variable. Al Kawaz et al. showed that patients with hyperoxia (> 120 mmHg) for more than 50% of the time were significantly more likely to die [15]. We previously conducted a randomized controlled trial (RCT) of hyperoxia during cardiac surgery. Even though the PaO2 was over 400 mmHg in the intervention group with the inspired fraction oxygen at 1, the incidence of an adverse outcome was similar to that observed in the control group [23], 24. The hyperoxia exposure time was approximately 100 min. Hence, the area under the curve for PaO2 might be a more appropriate measure of the duration and level of exposure to hyperoxia. In the present study, we assessed the degree of hyperoxia during the first 48 h on ECMO support; our observation of a significant association with mortality suggests that even though very short exposures (a few hours) are harmless, the cumulative time interval for a harmful effect is less than 48 h. This finding highlights the importance of avoiding hyperoxia from the initiation of ECMO support onwards.
Although many large RCTs have investigated hyperoxia in mechanically ventilated or perioperative ICU patients, a significant association with mortality has not been found [25–27]. This raises the question of why hyperoxia would be harmful in patients on VA-ECMO support only. The initial severity of the patient’s status might have a role. One can hypothesize that ROS production is more pronounced in the most critical patients exposed to hyperoxia. In a large RCT of hyperoxia in septic shock patients (the HYPERS2 trial), a post hoc analysis revealed that the mortality rate was higher in patients with hyperoxia (FiO2 = 1) and an arterial blood lactate level > 2 mmol l−1. This finding confirms that the most critically ill patients were the most vulnerable to hyperoxia. In addition to the production of free radicals, hyperoxia can dysregulate the immune response and induce immunodepression [28]. All of our study participants (Table 1) met the criteria for the international Sepsis-3 definition of septic shock (with no evidence of infection), with mean arterial blood lactate of 6 mmol l−1 (> 2 mmol l−1) and a high SAPS II value [29].
Our study had some limitations. Firstly (and as with previous studies), the lack of consensus definitions of hyperoxia and a hyperoxia threshold was problematic. Secondly, oxygen demand might vary greatly as a function of various metabolic variables (body temperature, oxygen consumption, hemoglobin level, atmospheric pressure, blood pH, etc.). Overall, the definition of a single oxygen threshold appears not to be realistic, and oxygen demand variables should be taken into account (e.g., lactate clearance and central venous blood oxygen saturation). A third limitation relates to our inclusion of patients with femoro-axillary vs. femorofemoral VA-ECMO configurations [30]. The blood delivered by ECMO is usually fully saturated in oxygen, and retrograde flow will usually occur in the aorta. However, the aortic transition point between the anterograde flow produced to the patient’s heart and the retrograde flow produced by femorofemoral ECMO is difficult to determine [31, 32] Harlequin syndrome might result in differential hypoxia in the right arm and the brain, with poorly saturated blood delivered by ejection from the heart [33, 34] This is why we performed sensitivity analysis (Additional file 4: Table S1) after the exclusion of patients on femoro-axillary ECMO support. The association between hyperoxia and death (according to the OR) was consistent with our primary findings and so confirmed that the putative effect of hyperoxia was not influenced by the VA-ECMO configuration. Lastly, the retrospective design of the present study was associated with an inherent risk of bias. We are aware that other explicative variables are missing to describe the 28-day mortality in spite of the propensity analysis. Notably, the medical history before ECMO cannulation describing how severe was the patient (i.e., lactate course, vasoactive-inotropic score, cardiac fractional ejection) would have been valuable. The magnitude of the daily PaO2 is around 20 mmHg (Table 2) which seems a too narrow range to explain the difference in the mortality rate. Although we included patients with a refractory cardiogenic shock, we cannot exclude heterogeneity in the population study with different demands in oxygen. Recent analysis from large cohorts showed that the question of oxygen is complex. Low oxygen level might be beneficial for some pathologies, while higher oxygen level seems safer in sepsis [35–37]. Thus, that hypothetic heterogeneity in patients suggests that a unique oxygen level cannot be applied to all the patients but that an individualized oxygen is promoted based on the monitoring of oxygen consumption (central venous saturation, arterial lactate, etc.). A recent study reported the feasibility of oxygen challenge to assess lung ability to transport oxygen and could help in the individualization of oxygen settings [38]. The authors showed that good responders to oxygen challenge had a better risk of survival.
Overall, our findings suggest that a large RCT is now warranted. The BLENDER trial (ClinicalTrials.gov identifier NCT03841084) should provide some answers. Our present results (including the sensitivity analysis) might help to better interpret the BLENDER trial’s data. Study recruitment is ongoing, with a planned sample size of 300 patients allocated to standard care (SpO2 target 92–96%) or interventional care (FiO2 = 1). The main endpoint is the number of ICU-free days by post-randomization day 60.
Conclusion
A high blood oxygen level during initial patient management with VA-ECMO was associated with a greater risk of 28-day mortality, in a dose-dependent manner. An ongoing RCT should provide answers on the management of oxygen levels during VA-ECMO.
Supplementary Information
Additional file 1.Fig. S1 : A Scatter plot examining the relationship between hemoglobin and maximal PaO2 at admission, with a fitted line representing the regression model and 95% confidence interval. B Scatter plot examining the relationship between ECMO flow rate and maximal PaO2 at admission, with a fitted line representing the regression model and 95% confidence interval.
Additional file 2 Fig. S1: Predicted probability of 28-day mortality according to mean daily peak PaO2 (A), absolute peak PaO2 (B), and overall mean PaO2 (C). Gray band indicates 95% confidence interval.
Additional file 3 Fig. S1: 28-day survival curve according to the first 48hrs admission hyperoxia range.
Additional file 4. Table S1. The association between hyperoxia and 28-day mortality after the exclusion of patients on femoro-axillary ECMO support, before and after adjustment.
Acknowledgements
They thank all the Amiens and Lille Hospital University for their support.
Abbreviations
- CS
Cardiogenic shock
- ECMO
Extracorporeal membrane oxygenation
- FiO2
Fraction of inspired oxygen
- INTERMACS
Interagency registry for mechanically assisted circulatory support
- IPTW
Inverse probability of treatment weighting
- PaO2
Partial pressure of oxygen in arterial blood
- SAPS II
Simplified Acute Physiology Score
- SaO2
Arterial oxygen saturation
- SCAI
Society for cardiovascular angiography and interventions
- SpO2
Peripheral oxygen saturation
- VA
Venoarterial
Author contributions
MDM, CB, and EROAA contributed to study design. AL and SR performed data acquisition. FJ, GL, VP, NR, CD, CD, TC, PH, MG, PB, YM, and HD analyzed and interpreted the data. JM performed statistical analysis. MDM and OAA drafted the manuscript. All authors performed revision for important intellectual content and read and approved the final manuscript.
Funding
None.
Availability of data and materials
Available on request.
Declarations
Ethics approval and consent to participate
The study was approved by the investigational review board at the French Society of Anesthesia, Intensive Care and Perioperative Medicine (Paris, France), on January 17th, 2022 (reference: CERAR IRB 00010254-2022-009). In accordance with French legislation, all datasets used in the present study were registered with the French National Data Protection Commission (Commission nationale de l'informatique et des libertés) (Paris, France; methodology MR-004; reference: 2208336v0 for Amiens University Medical Center, and DEC2015-14 for Lille University Medical Center).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, et al. Contemporary management of cardiogenic shock: a scientific statement from the american heart association. Circulation. 2017 doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 2.Smith M, Vukomanovic A, Brodie D, Thiagarajan R, Rycus P, Buscher H. Duration of veno-arterial extracorporeal life support (VA ECMO) and outcome: an analysis of the extracorporeal life support organization (ELSO) registry. Crit Care. 2017;21:45. doi: 10.1186/s13054-017-1633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 4.Aubron C, DePuydt J, Belon F, Bailey M, Schmidt M, Sheldrake J, et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care. 2016;6:97. doi: 10.1186/s13613-016-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang M, Ong BH, Hoo AEE, Gao F, Chao VTT, Lim CH, et al. Prognostic factors for survival after extracorporeal membrane oxygenation for cardiogenic shock. ASAIO J. 2020;66:141–145. doi: 10.1097/MAT.0000000000000984. [DOI] [PubMed] [Google Scholar]
- 6.Moussa MD, Soquet J, Lamer A, Labreuche J, Gantois G, Dupont A, et al. Evaluation of anti-activated factor X activity and activated partial thromboplastin time relations and their association with bleeding and thrombosis during veno-arterial ecmo support: a retrospective study. JCM. 2021;10:2158. doi: 10.3390/jcm10102158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer M, Young PJ, Laffey JG, Asfar P, Taccone FS, Skrifvars MB, et al. Dangers of hyperoxia. Crit Care. 2021;25:440. doi: 10.1186/s13054-021-03815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng Y-W, Mohammed A, Deatrick KB, Major T, Cheng D, Charpie I, et al. Differential effects of normoxic and hyperoxic reperfusion on global myocardial ischemia-reperfusion Injury. Semin Thorac Cardiovasc Surg. 2019;31:188–198. doi: 10.1053/j.semtcvs.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Cashen K, Reeder R, Dalton HJ, Berg RA, Shanley TP, Newth CJL, et al. Hyperoxia and hypocapnia during pediatric extracorporeal membrane oxygenation: associations with complications, mortality, and functional status among survivors. Pediatr Crit Care Med. 2018;19:245–253. doi: 10.1097/PCC.0000000000001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passmore MR, Ki KK, Chan CHH, Lee T, Bouquet M, Wood ES, et al. The effect of hyperoxia on inflammation and platelet responses in an ex vivo extracorporeal membrane oxygenation circuit. Artif Organs. 2020;44:1276–1285. doi: 10.1111/aor.13771. [DOI] [PubMed] [Google Scholar]
- 11.Galantowicz ME, Price M, Stolar CJH. Differential effects of alveolar and arterial oxygen tension on pulmonary vasomotor tone in ECMO-perfused, isolated piglet lungs. J Pediatr Surg. 1991;26:312–316. doi: 10.1016/0022-3468(91)90508-Q. [DOI] [PubMed] [Google Scholar]
- 12.Yen C-C, Chang W-H, Tung M-C, Chen H-L, Liu H-C, Liao C-H, et al. Lactoferrin protects hyperoxia-induced lung and kidney systemic inflammation in an in vivo imaging model of nf-κb/luciferase transgenic mice. Mol Imag Biol. 2020;22:526–538. doi: 10.1007/s11307-019-01390-x. [DOI] [PubMed] [Google Scholar]
- 13.Munshi L, Kiss A, Cypel M, Keshavjee S, Ferguson ND, Fan E. Oxygen thresholds and mortality during extracorporeal life support in adult patients*: Crit Care Med. 2017;45(12):1997–2005. doi: 10.1097/CCM.0000000000002643. [DOI] [PubMed] [Google Scholar]
- 14.Ishii J, Ohshimo S, Shime N. Is oxygenation really an intrinsic predictive factor of mortality in patients undergoing extracorporeal life support?: Crit Care Med. 2018;46(2):e181–e182. doi: 10.1097/CCM.0000000000002810. [DOI] [PubMed] [Google Scholar]
- 15.Al-Kawaz MN, Canner J, Caturegli G, Kannapadi N, Balucani C, Shelley L, et al. Duration of hyperoxia and neurologic outcomes in patients undergoing extracorporeal membrane oxygenation. Crit Care Med. 2021;49:e968–e977. doi: 10.1097/CCM.0000000000005069. [DOI] [PubMed] [Google Scholar]
- 16.Toulouse E, Lafont B, Granier S, Mcgurk G, Bazin J-E. French legal approach to patient consent in clinical research. Anaesth Crit Care Pain Med. 2020;39:883–885. doi: 10.1016/j.accpm.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, Kormos RL, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transp. 2009;28:535–541. doi: 10.1016/j.healun.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019; ccd.28329. [DOI] [PubMed]
- 19.Adult Mortality-Longitudinal Sample INSEE. Available from: https://www.insee.fr/en/metadonnees/source/serie/s1018?debut=0
- 20.Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592. doi: 10.1093/biomet/63.3.581. [DOI] [Google Scholar]
- 21.Winiszewski H, Guinot P-G, Schmidt M, Besch G, Piton G, Perrotti A, et al. Optimizing PO2 during peripheral veno-arterial ECMO: a narrative review. Crit Care. 2022;26:226. doi: 10.1186/s13054-022-04102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross P, Miller C, Sheldrake J, McGuiness W, Udy A, Burrell A. Hyperoxia in patients with cardiogenic shock after myocardial infarction supported with venoarterial extracorporeal membrane oxygenation. Aust Crit Care. 2021;34:55–59. doi: 10.1016/j.aucc.2020.04.154. [DOI] [PubMed] [Google Scholar]
- 23.Abou-Arab O, Huette P, Martineau L, Beauvalot C, Beyls C, Josse E, et al. Hyperoxia during cardiopulmonary bypass does not decrease cardiovascular complications following cardiac surgery: the CARDIOX randomized clinical trial. Intensive Care Med. 2019;45:1413–1421. doi: 10.1007/s00134-019-05761-4. [DOI] [PubMed] [Google Scholar]
- 24.Abou-Arab O, Huette P, Guilbart M, Dupont H, Guinot P-G. Hyperoxia during cardiopulmonary bypass does not increase respiratory or neurological complications: a post hoc analysis of the CARDIOX study. Br J Anaesth. 2020;125(5):e400–e401. doi: 10.1016/j.bja.2020.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382:999–1008. doi: 10.1056/NEJMoa1916431. [DOI] [PubMed] [Google Scholar]
- 26.Clinical Trials Group Conservative oxygen therapy during mechanical ventilation in the ICU. New Engl J Med. 2020;382(11):989–998. doi: 10.1056/NEJMoa1903297. [DOI] [PubMed] [Google Scholar]
- 27.Lassen ML, Risgaard B, Baekgaard JS, Rasmussen LS. Determining a safe upper limit of oxygen supplementation for adult patients: a systematic review. BMJ Open. 2021;11:e045057. doi: 10.1136/bmjopen-2020-045057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sjöberg F, Singer M. The medical use of oxygen: a time for critical reappraisal. J Intern Med. 2013;274:505–528. doi: 10.1111/joim.12139. [DOI] [PubMed] [Google Scholar]
- 29.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moussa MD, Rousse N, Arab OA, Lamer A, Gantois G, Soquet J, et al. Subclavian versus femoral arterial cannulations during extracorporeal membrane oxygenation: a propensity-matched comparison. J Heart Lung Transpl. 2022;41(5):608–618. doi: 10.1016/j.healun.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Buchtele N, Staudinger T, Schwameis M, Schörgenhofer C, Herkner H, et al. Feasibility and safety of watershed detection by contrast-enhanced ultrasound in patients receiving peripheral venoarterial extracorporeal membrane oxygenation: a prospective observational study. Crit Care. 2020;24:126. doi: 10.1186/s13054-020-02849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honore PM, Barreto Gutierrez L, Kugener L, Redant S, Attou R, Gallerani A, et al. Risk of harlequin syndrome during bi-femoral peripheral VA-ECMO: should we pay more attention to the watershed or try to change the venous cannulation site? Crit Care. 2020;24:450. doi: 10.1186/s13054-020-03168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avgerinos DV, DeBois W, Voevidko L, Salemi A. Regional variation in arterial saturation and oxygen delivery during venoarterial extracorporeal membrane oxygenation. J Extra Corpor Technol. 2013;45:183–186. [PMC free article] [PubMed] [Google Scholar]
- 34.Pasrija C, Bedeir K, Jeudy J, Kon ZN. Harlequin syndrome during venoarterial extracorporeal membrane oxygenation. Radiol Cardiothorac Imaging. 2019;1:e190031. doi: 10.1148/ryct.2019190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young P, Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, et al. Conservative oxygen therapy for mechanically ventilated adults with suspected hypoxic ischaemic encephalopathy. Intensive Care Med. 2020;46:2411–2422. doi: 10.1007/s00134-020-06196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young P, Mackle D, Bellomo R, Bailey M, Beasley R, et al. Conservative oxygen therapy for mechanically ventilated adults with sepsis: a post hoc analysis of data from the intensive care unit randomized trial comparing two approaches to oxygen therapy (ICU-ROX) Intensive Care Med. 2020;46:17–26. doi: 10.1007/s00134-019-05857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klitgaard TL, Schjørring OL, Lange T, Møller MH, Perner A, Rasmussen BS, et al. Lower versus higher oxygenation targets in critically ill patients with severe hypoxaemia: secondary Bayesian analysis to explore heterogeneous treatment effects in the Handling Oxygenation Targets in the Intensive Care Unit (HOT-ICU) trial. Br J Anaesth. 2022;128:55–64. doi: 10.1016/j.bja.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong L, Zhang X, Liang F, Yu X, Yang T, Li L. Prognostic value of oxygen challenge test for patients with cardiogenic shock receiving extracorporeal membrane oxygenation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017;29:1102–1106. doi: 10.3760/cma.j.issn.2095-4352.2017.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1.Fig. S1 : A Scatter plot examining the relationship between hemoglobin and maximal PaO2 at admission, with a fitted line representing the regression model and 95% confidence interval. B Scatter plot examining the relationship between ECMO flow rate and maximal PaO2 at admission, with a fitted line representing the regression model and 95% confidence interval.
Additional file 2 Fig. S1: Predicted probability of 28-day mortality according to mean daily peak PaO2 (A), absolute peak PaO2 (B), and overall mean PaO2 (C). Gray band indicates 95% confidence interval.
Additional file 3 Fig. S1: 28-day survival curve according to the first 48hrs admission hyperoxia range.
Additional file 4. Table S1. The association between hyperoxia and 28-day mortality after the exclusion of patients on femoro-axillary ECMO support, before and after adjustment.
Data Availability Statement
Available on request.