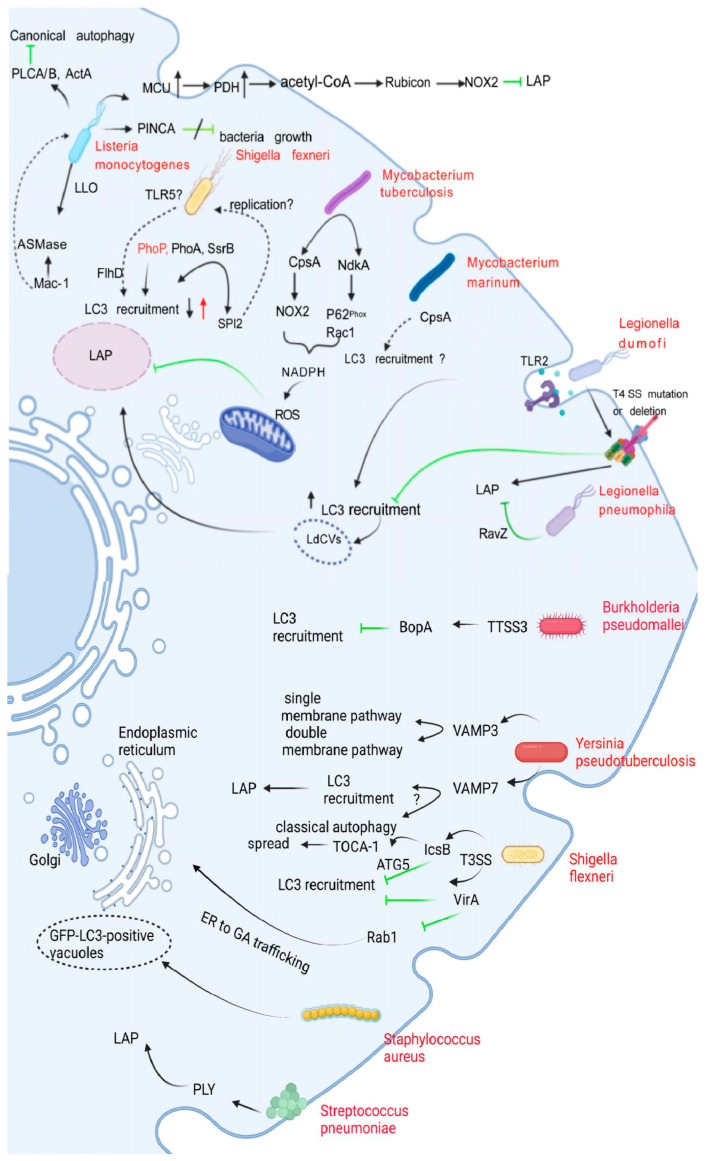
Figure 3.
Interactions of bacteria with LAP. This figure shows the correlation between several bacteria and LAP (black arrows). Green arrows indicate inhibition. Dotted arrows represent interactions that are not yet confirmed. L. monocytogenes uses its own virulence factors, PLCA/B and ActA, to protect it from being recognized, killed, and degraded by autophagy targets. PINCA did not play a substantial role in anti-L. monocytogenes and did not inhibit bacterial growth. L. monocytogenes promotes the uptake of mtCa2+ by regulating MCU to enhance PDH activity, thereby inducing the production of acetyl-CoA. Acetyl-CoA acetylates Rubicon, resulting in decreased Rubicon content and inhibiting the interaction of Rubicon with the NOX2 complex, thereby inhibiting the formation of LAP. The interaction of L. monocytogenes with Mac-1 induces ASMase-mediated changes in membrane lipid composition and converts sphingomyelin to ceramide and phosphorylated choline. The deletion of the PhoP and PurA virulence factors in S. typhimurium increased and decreased LC3 recruitment, respectively, and SsrB is part of the bacterial regulatory system that controls the expression of SPI2 effector molecules and is required for the maintenance of Salmonella-containing vacuoles (SCV). However, Rubicon knockout did not affect the survival of mutant SsrB strains, possibly because SPI2 is related to bacterial replication in vivo. CpsA acts upstream of NOX2 by blocking the recruitment of NOX2 to the phagosome; M. tuberculosis also secretes a virulence factor called NdkA. The presence of NdkA reduces the uptake of p67phox and Rac1 to the phagosome and interferes with NADPH oxidation. The enzyme complex generates ROS and may destroy LAP. LAP binds LC3 to LdCVs in a Dot/Icm T4SS-dependent manner, leading to bacterial degradation, a process that requires TLR2. L. pneumophila can irreversibly uncouple the conjugation function of LC3 and phosphatidylethanolamine through RavZ, and can also block LAP induced by its infection. BopA, an important effector protein encoded by the TTSS3 gene of B. pseudomallei, inhibits LC3 recruitment. The high and low expression of VAMP3 in Y. pseudotuberculosis can localize in autophagic vesicles of monolayer and bilayer membranes, respectively. VAMP7 inhibits the maturation of YCVs and disrupts LAP and autophagy, but the mechanism remains unclear. The spread of S. flexneri is dependent on the function of T3SS. IcsB blocks the recruitment of LC3 by blocking the binding of the ATG5 to the surface protein VirG of S. flexneri. IcsB is also thought to recruit the actin-associated protein TOCA-1, promoting cell-to-cell spread. Likewise, VirA prevents LC3 recruitment to S. flexneri-containing vesicles. Specifically, the inactivation of Rab1 by VirA inhibits the effect of ER on GA transport, but it remains unclear how Rab1 is involved in LC3 recruitment to S. flexneri-containing vacuoles. S. aureus was found to establish an intracellular niche in neutrophils, the mechanism of which has not been elucidated. S. pneumoniae-induced LC3 recruitment is dependent on pneumolysin.