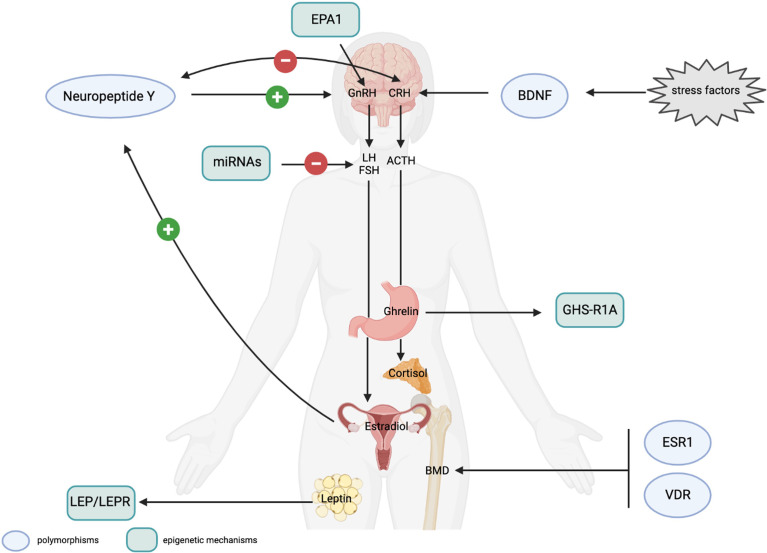
Figure 2.
(Epi)genetic mechanisms possibly involved in FHA development and FHA-related long-term consequences. Genetic mechanisms. Polymorphisms in the Neuropeptide Y (NPY) and BDNF genes affect stress response. The NPY positively controls GnRH secretion in presence of adequate levels of estrogen and has an anxiolytic effect by counteracting CRH activity. CRH, itself, downregulates the expression of the NPY. NPY polymorphisms have been associated to resilience or stress-sensitive phenotypes. BDNF polymorphisms are suggested to affect neuroplasticity and stress responses. Polymorphisms in the estrogen receptor (ESR1) and in the vitamin D receptor (VDR) genes influence bone mineral density (BMD) and may be associated to osteopenia and osteoporosis, consequent to prolonged hypoestrogenism. Epigenetic mechanisms. The EPA1 transcription factor controls GnRH expression and a 5’-UTR polymorphism has been associated with a higher risk of amenorrhea in animal models. Altered methylation levels of the LEP and LEPR genes have been associated with the effect of leptin, produced by adipocytes on the HPG axis and on the personal response to psychotherapeutic treatment in AN patients. Methylation of the ghrelin receptor gene (GHS-R1A) are thought to be involved in ghrelin resistance affecting GnRH secretion. Specific miRNAs have been reported to control the post-transcriptional expression of LH and FSH, and to be a promising peripheral biomarkers to control the effect of hormonal therapy in FHA women. Light blue circles indicate polymorphic variants in genes possibly associated to response to stress or long-term consequences in FHA women; light green rectangles indicate the epigenetic mechanisms (including transcription factors, miRNA and methylation) that can play a role in the regulation of the HPG axis and in FHA development (created with BioRender.com).