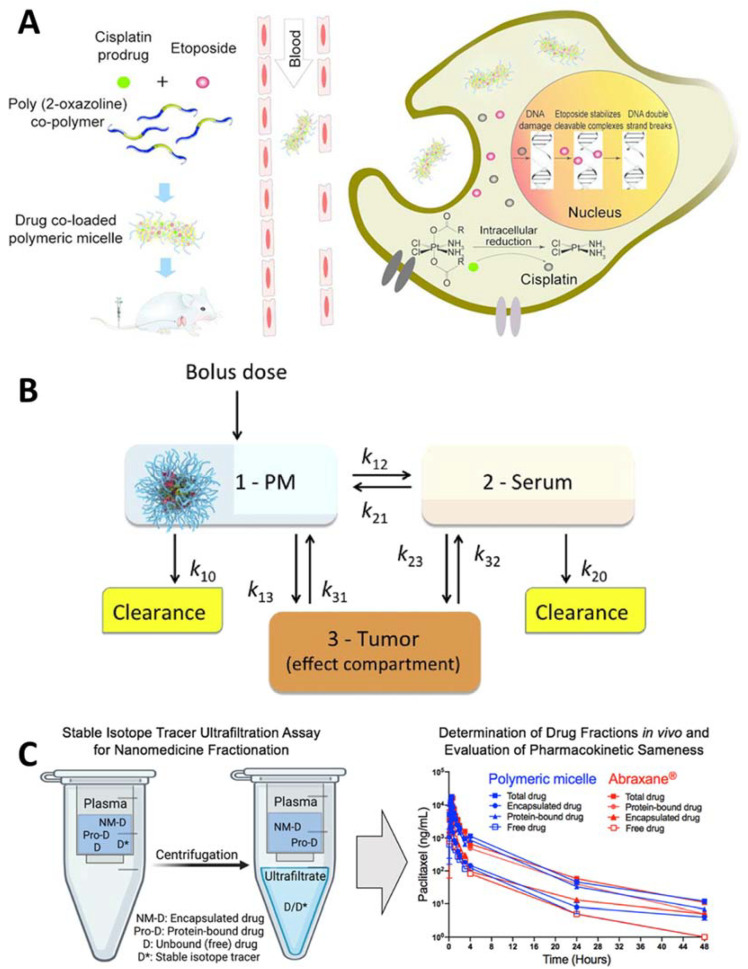
Figure 3.
Examples of the application of Poly(2-oxazoline) (POx)-based polymeric micelles and the assessment of their pharmacokinetics (PK) and bioequivalence profiles. In (A) poly(2-methyl-2-oxazoline-block-2-butyl-2-oxazoline-block-2-methyl-2-oxazoline) (P(MeOx-b-BuOx-b-MeOx) is used with an alkylated cisplatin prodrug to enable co-formulation of etoposide (ETO) and platinum drug combination (“EP/PE”) in a single high-capacity vehicle to improve the treatment of small cell lung cancer (SCLC). The drugs co-loading in the micelles result in a slowed-down release, improved pharmacokinetics, and increased tumor distribution of both drugs. Reprinted with permission from [58]. Copyright 2018 American Chemical Society. In (B) a three-compartmental model describing the PMs drug delivery to a tumor. The drug is administered as bolus in the form of PMs (1) and is subsequently distributed between the serum (2) and tumor (3) compartments. The PK constants correspond to: k12—rate of drug transfer from PMs to serum; k21—rate of drug re-capture from serum to PMs; k13—rate of transfer (permeability) of the micellar drug to tumor; k23—rate of transfer of the serum-bound drug to tumor; k31 and k32—rates of drug reabsorption from tumor to PMs and serum, respectively; and k10 and k20—micellar and serum-bound drug clearances, respectively. The model assumes that the drug solubility in blood is very low and the free drug form in the blood is, therefore, neglected. Reprinted from [59], Copyright 2018, with permission from Elsevier. In (C) a comprehensive preclinical assessment of the poly (2-oxazoline)-based polymeric micelle of paclitaxel (PTX) (POXOL hl-PMs), including bioequivalence comparison to the clinically-approved paclitaxel nanomedicine, Abraxane®. Reprinted from [67], Copyright 2018, with permission from Elsevier.