Table 3.
The substrate scope of the AcOH-catalyzed three-component reaction between phenacyl alcohols 14–17, oxoacetonitriles 2, 13, and 18–20, and primary amines 3, 5, 6, 10, 11, and 21 for the selective synthesis of 3-cyanopyrroles 14a–e, 15a, 16a–b, and 17a–b [a].
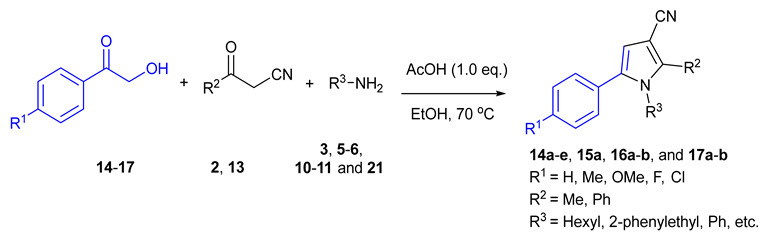
| No. | α-Hydroxyketon | Oxoacetonitrile | Amine | 3-Cyanopyrrole | Yield [b] |
|---|---|---|---|---|---|
| 1 |
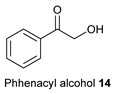
|

|

|
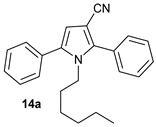
|
90 |
| 2 |

|

|

|

|
88 |
| 3 |

|
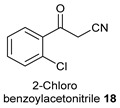
|

|

|
86 |
| 4 |

|

|

|

|
80 |
| 5 |

|

|

|

|
77 |
| 6 |

|

|

|

|
74 |
| 7 |

|

|

|

|
82 |
| 8 |

|

|

|

|
70 |
| 9 |

|

|

|

|
84 |
| 10 |

|

|

|
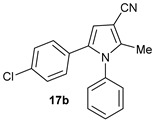
|
70 |
[a] A mixture of phenacyl alcohols 14–17 (1.0 mmol), oxoacetonitriles 2, 13 and 18–20 (1.0 mmol), and primary amines 3, 5, 6, 10, 11 and 21 (1.1 mmol) were stirred in EtOH (3 mL) at 70 °C for 3 h. [b] Isolated yields.
