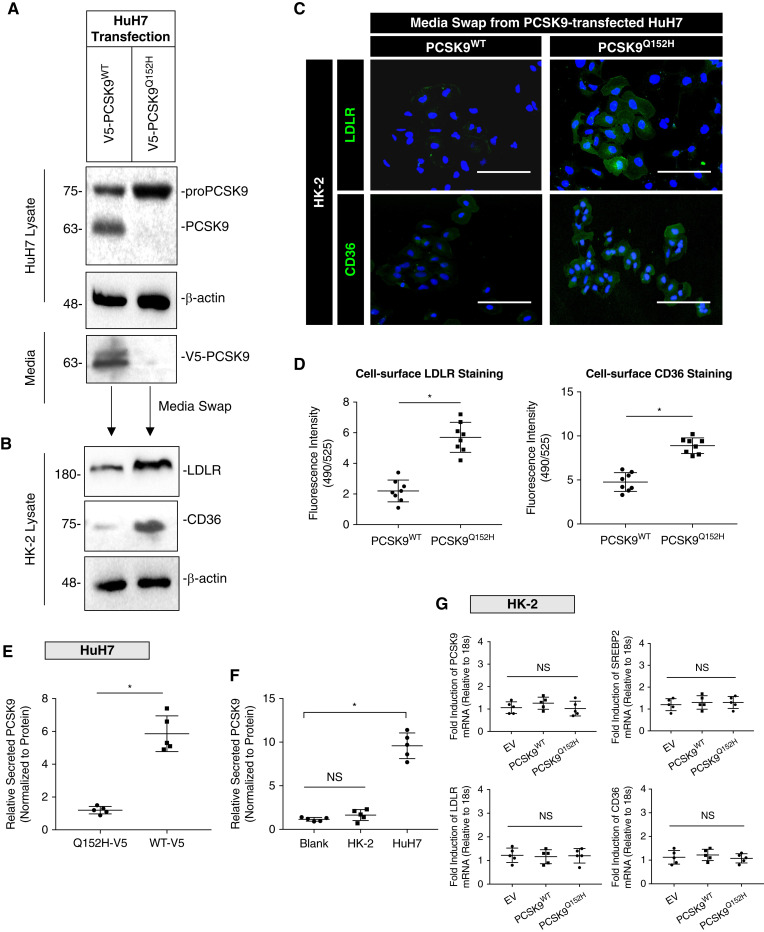
Figure 1.
Secreted PCSK9 regulates the LDL receptor (LDLR) and cluster of differentiation 36 (CD36) in cultured renal cells. (A) Whole-cell lysates and media immunoblotted for PCSK9 from HuH7 cells transfected with either PCSK9WT or PCSK9Q152H expression plasmids. (B) Relative protein expression of CD36 and LDLR in whole-cell lysates from HK-2 cells exposed to media harvested from HuH7 cells. (C and D) Immunofluorescence microscopy staining for surface CD36 and LDLR in HK-2 cells post media swap (*P<0.05). (E) Relative secreted PCSK9 measured in HuH7 cells transfected with either PCSK9WT or PCSK9Q152H after 24 hours (*P<0.05). (F) Secreted PCSK9 was also measured in HuH7 and HK-2 cells to confirm that renal cells secrete less PCSK9 relative to hepatocytes in vitro (*P<0.05, NS). (G) mRNA transcript levels of PCSK9, SREBP2, LDLR, and CD36 in HK-2 cells exposed to harvested media from HuH7-transfected cells confirming that modulation of surface receptors on HK-2 cells were through exogenously added PCSK9 from hepatocytes (NS). Data are represented as the mean, and errors are represented as the SD. Differences between groups were determined using t tests or ANOVAs. Scale bars, 200 µm.