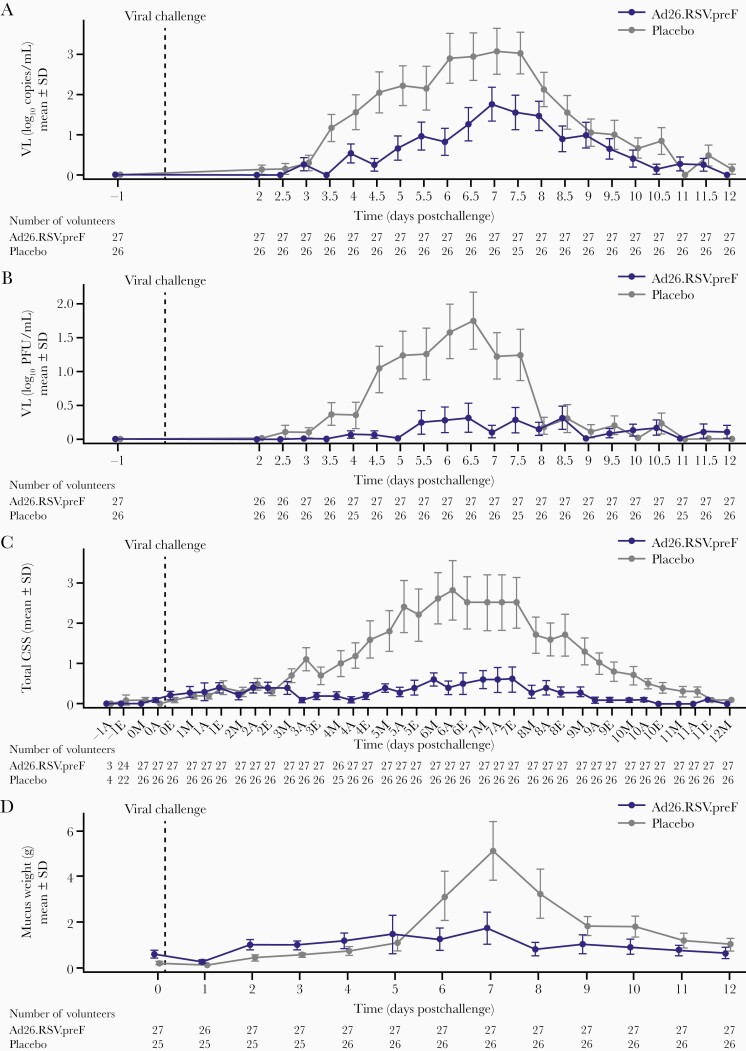
Figure 2.
Efficacy as determined by viral load as determined by real-time polymerase chain reaction (rt-PCR) and quantitative culture, and disease severity as determined by total clinical symptom score (CSS) and mucus weight over time following intranasal viral challenge with respiratory syncytial virus subtype A (RSV-A) after receiving immunization with either Ad26.RSV.preF or placebo intramuscularly (intent-to-treat challenge population). A, Viral load as determined by rt-PCR. B, Viral load as determined by quantitative culture over time. C, Total CSS over time. On the x-axis, A indicates afternoon; E, evening; M, morning. D, Total mucus weight over time. Viral load as determined by rt-PCR, viral load as determined by quantitative culture, CSS, and mucus weight are shown from viral challenge at day 0 until 12 days postchallenge in the intent-to-treat challenge set. From days 2 to 12 postchallenge, nasal wash samples were collected (twice daily from days 2 to 11, once daily on day 12), and symptom score cards were completed by volunteers (thrice daily from days 2 to 11, once daily on day 12) and reviewed by the attending physician. Mucus weights were determined every 24 hours. Placebo and Ad26.RSV.preF were measured at the same timepoints, jitter was applied to ensure both Ad26.RSV.preF and placebo means, and confidence intervals are visible even if close to each other. Abbreviations: CSS, clinical symptom score; SD, standard deviation; VL, viral load.