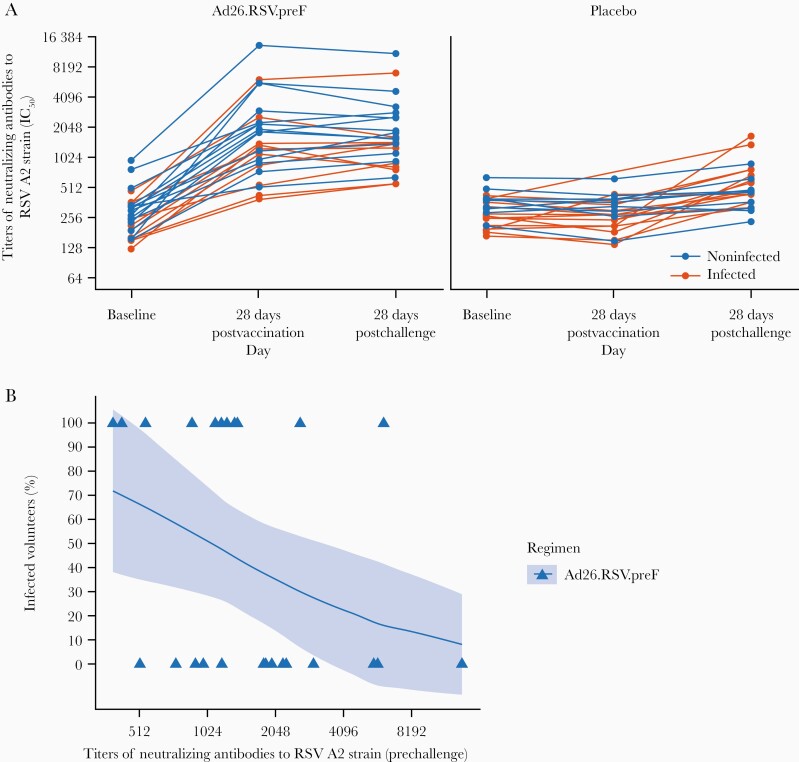
Figure 4.
Titers of neutralizing antibodies to respiratory syncytial virus (RSV) A2 strain over time, and proportion of infected volunteers vs prechallenge titers of neutralizing antibodies to RSV A2 strain following immunization with either Ad26.RSV.preF or placebo intramuscularly (per-protocol immunogenicity set). A, Titers of neutralizing antibodies to RSV A2 strain are shown from baseline to 28 days postchallenge in the per-protocol immunogenicity set. Geometric mean titers with 95% confidence intervals (CIs) are shown. The majority of the volunteers had their 28-day postimmunization sample taken between 22 and 33 days after immunization. One volunteer had 2 prechallenge samples taken at day 45 and day 70; these 2 samples are not part of the analysis. Infection was defined based on the symptomatic and asymptomatic definition: if a volunteer had ≥2 quantifiable real-time polymerase chain reaction (rt-PCR) measurements above the lower limit of quantification (LLOQ). B, Infected and noninfected volunteers receiving Ad26.RSV.preF were plotted vs their prechallenge titers of neutralizing antibodies to RSV A2 strain. Generalized linear regression was used to model the probability of those in the Ad26.RSV.preF group being infected using the log-transformed prechallenge titers of neutralizing antibodies to RSV A2 strain as an independent variable. The solid lines represent the estimated probability of being infected and the shaded area around it represents the 95% CI. Infection was defined based on the symptomatic and asymptomatic definition: if a volunteer had ≥2 quantifiable rt-PCR measurements above the LLOQ. Abbreviations: IC50, half-maximal inhibitory concentration; RSV, respiratory syncytial virus.