Optimization of reaction conditions for the oxidation of benzyl alcohol in terms of solvent and oxidanta.
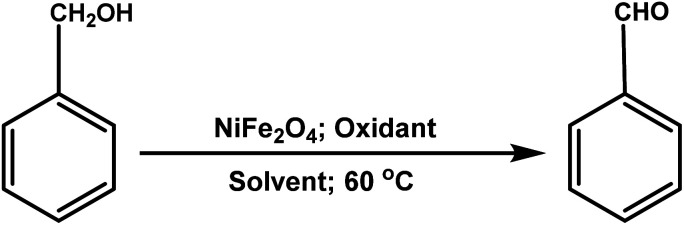
| ||||
|---|---|---|---|---|
| Entry | Solvent (mL) | Oxidant (mL) | Time (h) | Yieldc (%) |
| 1 | — | O2 | 6 | Trace |
| 2 | — | H2O2 (0.5) | 6 | Trace |
| 3 | — | TBHP (0.05) | 6 | 30 |
| 4b | — | TBHP (0.05) | 6 | Trace |
| 5 | C2H5OH (3.0) | TBHP (0.05) | 6 | Trace |
| 6 | CH3OH (3.0) | TBHP (0.05) | 6 | 15 |
| 7 | 2-Propanol (3.0) | TBHP (0.05) | 6 | 42 |
| 8 | CH3CN (3.0) | TBHP (0.05) | 3 | 85 |
| 9 | H2O (3.0) | TBHP (0.05) | 6 | Trace |
Benzyl alcohol = 1.0 mmol; catalyst = 10 mg (0.04 mmol).
No catalyst was used.
Isolated yield.
