Catalytic performance in the oxidation of benzylic and aliphatic alcoholsa.
| Entry | Alcohol | Product | Time (h) | Conversionb (%) | Specific activity (mmol g−1 h−1) | TONc | TOFd (h−1) |
|---|---|---|---|---|---|---|---|
| 1 |
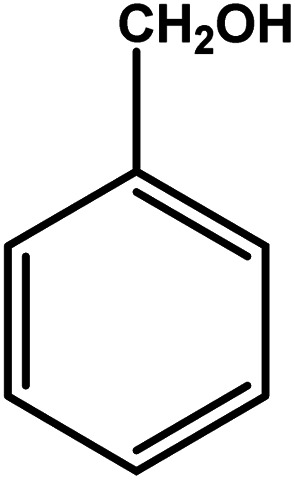
|
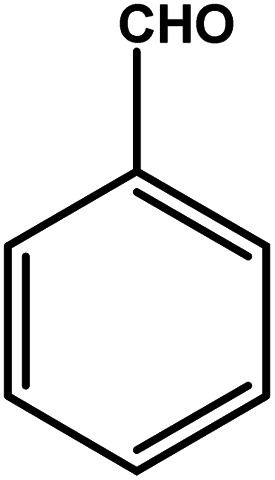
|
3 | 85 | 28.3 | 21.25 | 7.1 |
| 2 |
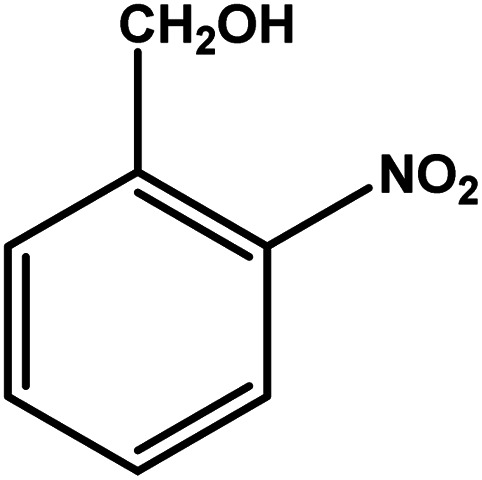
|
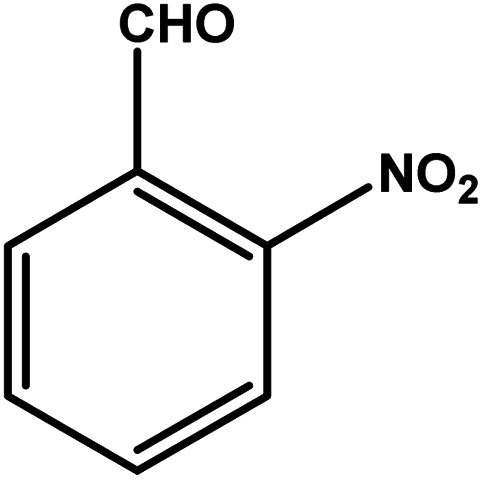
|
3 | 76 | 25.3 | 19.0 | 6.3 |
| 3 |
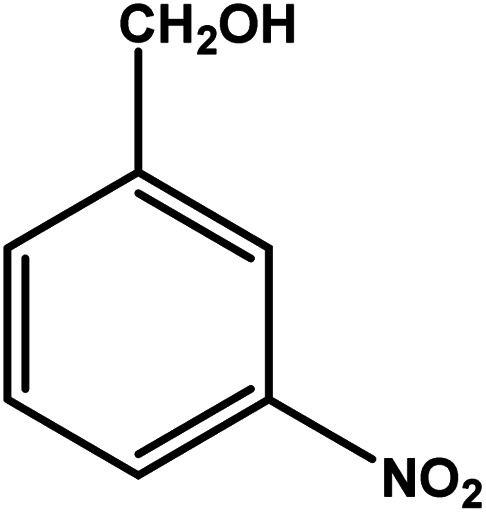
|
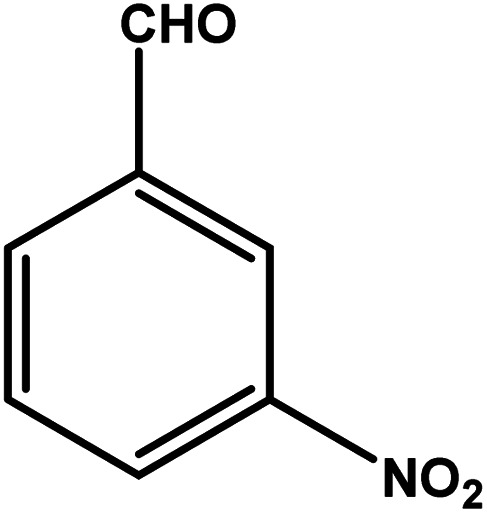
|
3 | 70 | 23.3 | 17.5 | 5.8 |
| 4 |
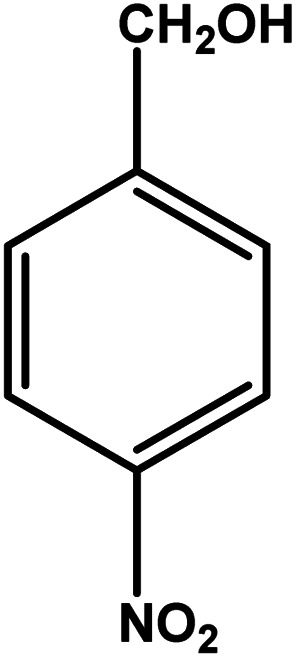
|
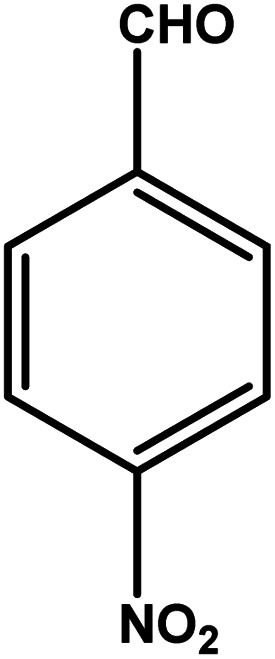
|
3 | 88 | 29.3 | 22.0 | 7.3 |
| 5 |
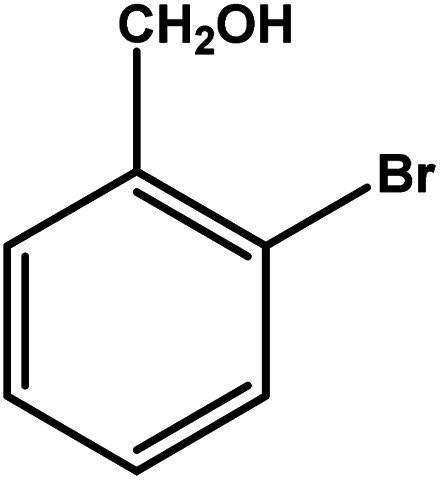
|
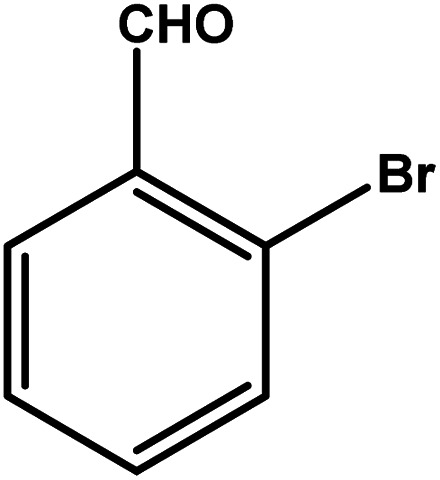
|
3.5 | 75 | 21.4 | 18.75 | 5.3 |
| 6 |
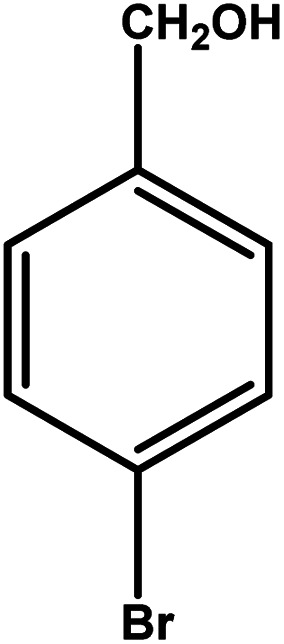
|
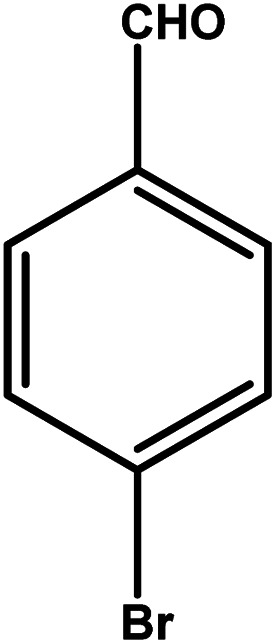
|
2 | 88 | 44.0 | 22.0 | 11.0 |
| 7 |
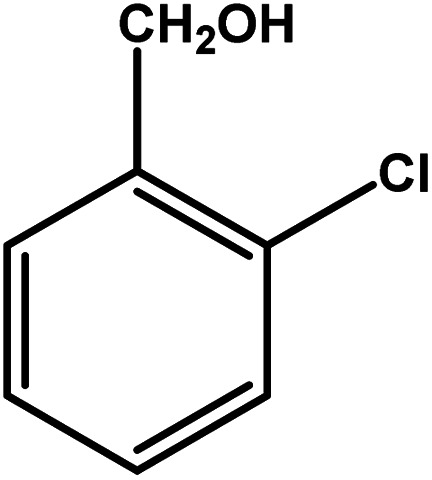
|
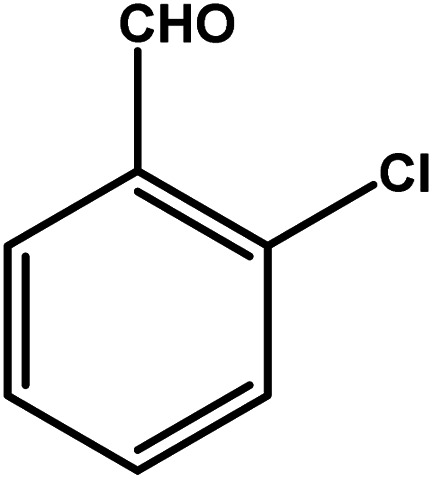
|
4 | 68 | 17.0 | 17.0 | 4.3 |
| 8 |
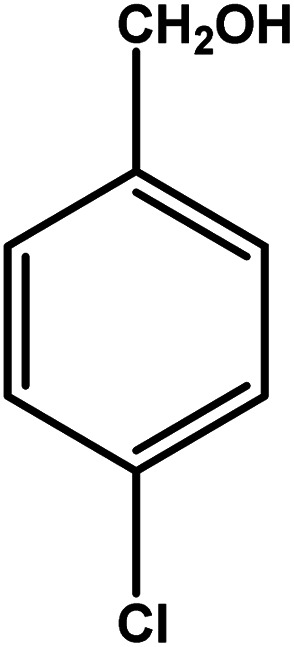
|
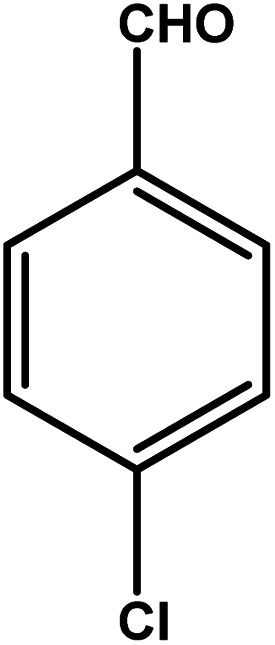
|
3 | 81 | 27.0 | 20.25 | 6.8 |
| 9 |
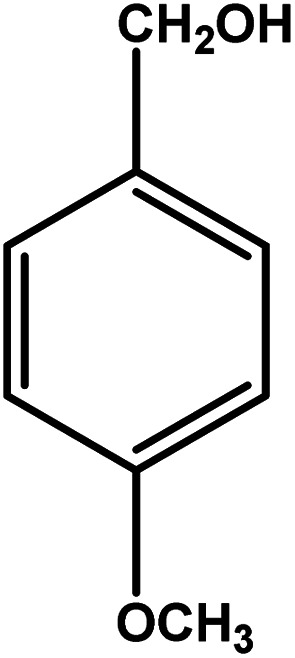
|
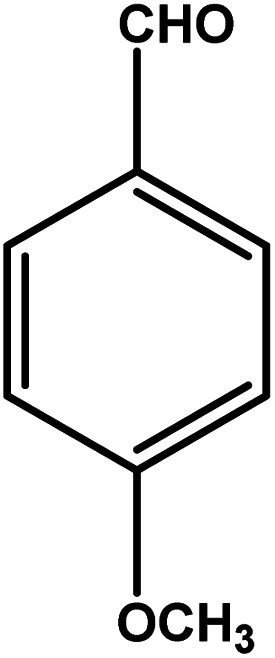
|
3 | 79 | 26.3 | 19.75 | 6.6 |
| 10 |
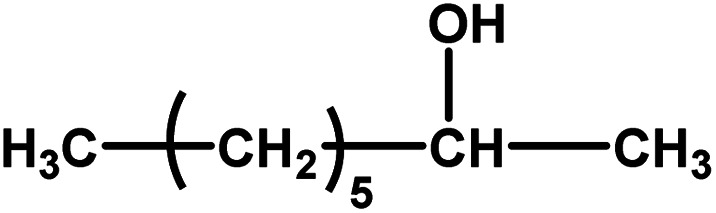
|
No reaction | 24 (observation) | — | — | — | — |
| 11 |
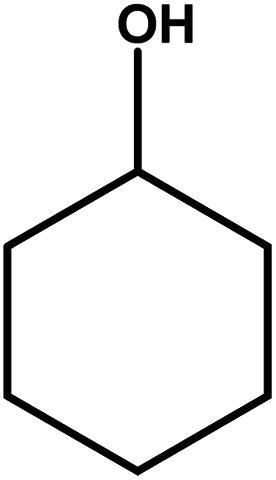
|
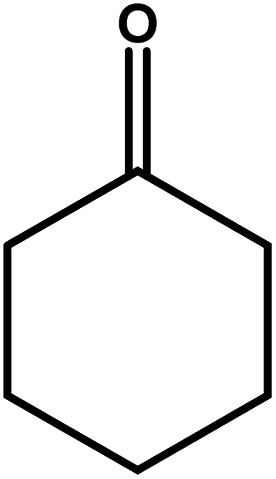
|
18–24 | Trace amount | — | — | — |
| 12 |
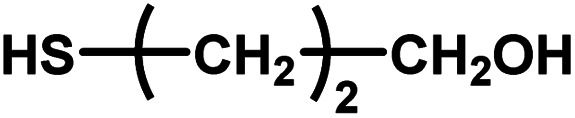
|
No reaction | 24 (observation) | — | — | — | — |
| 13 |
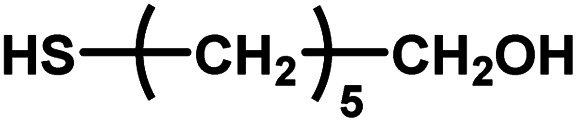
|
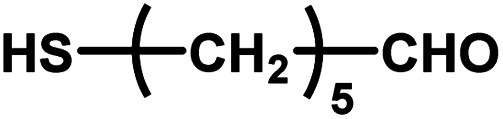
|
12–24 | ∼30 | — | — | — |
Reaction conditions: benzyl alcohol: 1 mmol; TBHP: 0.5 mmol; NiFe2O4: 10 mg; solvent: CH3CN; temperature: 60 °C.
Isolated yield.
TON: turnover number = number of moles of substrate consumed/number of moles of catalyst.
TOF: turnover frequency = TON/time of reaction in h.
