Abstract
Purpose
To explore the benefit yielded by radiotherapy (RT), we report a series of metastatic renal cell carcinoma (RCC) patients treated with concomitant RT plus Nivolumab.
Methods/patients
Patients undergoing Nivolumab treatment plus concomitant RT (ablative or palliative) were included. RT was defined Ablative if >5 Gy/fraction were delivered.
Results
Ablative RT intent was the only independent predictor of both progression free and overall survival (HR 3.51, 95% CI 1.6–7.5, p = 0.0012 and HR 2.8, 95% CI 0.99–8.07, p = 0.05, respectively).
Conclusion
Ablative RT may improve oncologic outcomes in selected patients with metastatic RCC treated with Nivolumab as compared to palliative RT.
Keywords: Metastatic renal cell carcinoma, SBRT, Stereotactic ablative radiotherapy
Introduction
Immune checkpoint inhibition (ICI) is one of the cornerstones of the contemporary treatment of metastatic renal cell carcinoma (RCC), both in first [1–4] and second line settings [5, 6]. Notably, the latest European Association of Urology (EAU) Guidelines recommend to offer stereotactic body radiotherapy (SBRT) to patients with metastatic disease and favorable disease factors to control local symptoms [6]. Interestingly, pre-clinical evidence highlighted a biological rationale for the potential added benefit of SBRT on top of ICI, while clinical evidence of such a synergistic effect is still controversial [7]. Herein, we report a retrospective multicentric series of metastatic RCC patients treated with concomitant radiotherapy (RT) plus ICI (Nivolumab as I-, II- or III-line therapy), aiming to compare the benefit yielded by SBRT vs palliative RT in this setting.
Material and Methods
After Ethical Committee approval, data from patients treated between June 2016 and November 2020 at 3 referral centers were retrospectively collected. All included patients gave written consent. Patients with either synchronous or metachronous metastatic RCC undergoing ICI with Nivolumab as I- or II-/III-line treatment plus concomitant RT were included. RT was considered concomitant if it was administered within ≤ 4 months before the start or after the end of Nivolumab treatment.
RT was administered with either an ablative (SBRT) or palliative treatment purpose based on each patient’s characteristics (i.e., metastatic site and technical feasibility of an ablative approach) according to physicians’ discretion. RT regimens were chosen according to adjacent critical organs at risk, and were defined as ablative if ≥ 5 Gy/fraction were delivered [8]. SBRT included regimens providing total doses of 18–54 Gy in 1–8 fractions, while 20–39 Gy in 5–15 fractions were administered for palliative treatments. Doses and fractionation schedules were prescribed according to clinician choice in the ablative group aiming to administer the maximum equivalent dose to the target respecting organ at risk dose constraints, with at least 5 GY per fraction. In patients undergoing palliative treatment, dose and fractionation schedules were delivered aiming to administered a dose at least equivalent to 20 Gy in 5 fraction considering an alpha/beta ratio of 10 (Fig. 1). Volumetric Modulated Arc Therapy and 3d Conformal technique were used for palliative purpose, Ablative treatment were administered also with CyberknifeR robotic system or GammaknifeR (Table 1). Detail for doses and fractionation schedules for 9 brain metastases treated are summarized in Table 2.
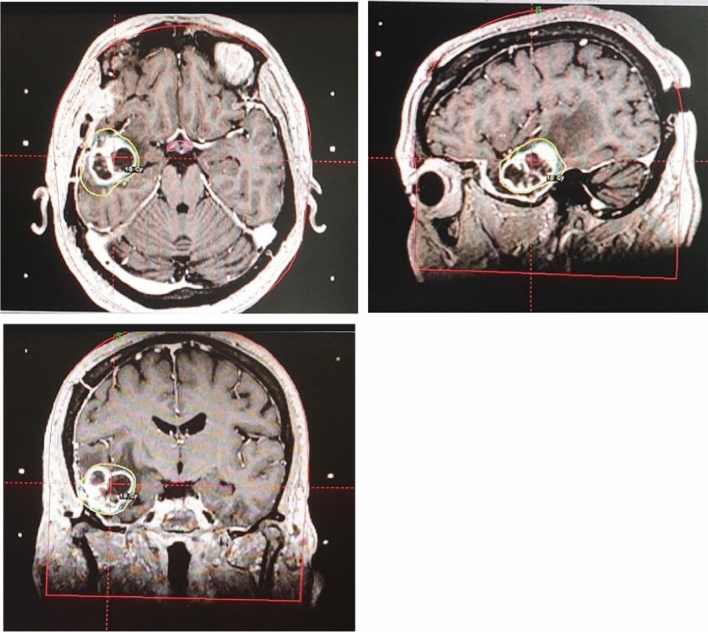
Fig. 1.
Example of a metastatic brain lesion treated with ablative intent (18 Gy in 1 fraction)
Table 1.
Main dose/fractionation schedules and techniques used for 52 treated lesions
| Dose/fractionation | n (%) |
|---|---|
| Ablative treatments | 28 (53.8) |
| 44 Gy/8 fractions | 1 (1.9) |
| 36 Gy/6 fractions | 2 (3.9) |
| 45 Gy/5 fractions | 1 (1.9) |
| 40 Gy/5 fractions | 1 (1.9) |
| 30 Gy/5 fractions | 10 (19.3) |
| 25 Gy/5 fractions | 1 (1.9) |
| 54 Gy/3 fractions | 1 (1.9) |
| 45 Gy/3 fractions | 1 (1.9) |
| 30 Gy/3 fractions | 1 (1.9) |
| 18 Gy/3 fractions | 1 (1.9) |
| 24 Gy/1 fraction | 8 (15.4) |
| Techniques | |
| VMAT | 20 (38.5) |
| GK | 6 (11.5) |
| CK | 2 (3.8) |
| Palliative treatments | 24 (46.2) |
| 37.5 Gy/15 fractions | 1 (1.9) |
| 39 Gy/13 fractions | 1 (1.9) |
| 30 Gy/10 fractions | 5 (9.7) |
| 20 Gy/5 fractions | 17 (32.7) |
| Techniques | |
| VMAT | 4 (7.7) |
| 3dCRT | 20 (38.5) |
VMAT volumetric modulated arc therapy, GK Gammaknife radiosurgery, CK CyberknifeR robotic radiotherapy, 3dCRT three dimensional conformal radiotherapy
Table 2.
Main dose/fractionation schedules and techniques used for 9 brain treated lesions
| Dose/fractionation | n (%) |
|---|---|
| Brain treatments | 9 (17.3) |
| 24 Gy/single fraction | 5 (9.6) |
| 21 Gy/single fraction | 1 (1.9) |
| 20 Gy/single fraction | 1 (1.9) |
| 18 Gy/single fraction | 1 (1.9) |
| 18 Gy/3 fractions | 1 (1.9) |
| Techniques | |
| VMAT | 2 (3.8) |
| GK | 6 (11.5) |
| CK | 1 (1.9) |
VMAT volumetric modulated arc therapy, GK Gammaknife radiosurgery, CK CyberknifeR robotic radiotherapy
The primary outcomes of the study were overall survival (OS) and progression-free survival (PFS). Overall survival (OS) was defined as time between Nivolumab Start and death. Progression-free survival (PFS) was defined as time between Nivolumab start and end. Local control outcomes and adverse events according to Common Terminology Criteria for Adverse Events were collected and reported. Kaplan–Meier analysis was performed to explore the correlation between clinical outcomes, initial staging (metachronous vs synchronous metastases) and RT intent (ablative vs palliative only). Cox proportional hazard model was used for multivariate analysis, including stage at diagnosis (metastatic vs non-metastatic) and RT goal (ablative vs palliative). Chi square test was used to compare local control in patients treated with ablative and palliative intent.
Results
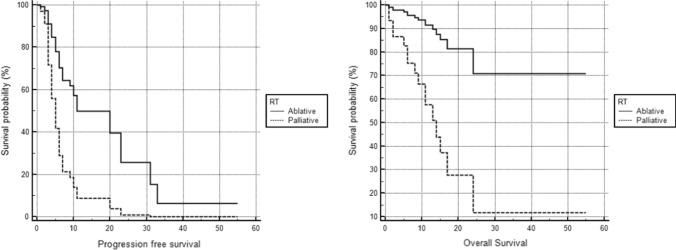
Overall, 40 patients with 52 metastatic lesions were included, 19 (47.5%) were treated with Nivolumab plus SBRT, and 21 (52.5%) with Nivolumab plus palliative RT. Baseline patient characteristics are summarized in Table 3. The proportion of patients with IMDC poor/intermediate risk was comparable between the study groups (p = 0.43). Among patients with synchronous metastatic RCC (n = 15), 14 (93.3%) underwent cytoreductive nephrectomy (CN) before ICI plus RT treatment. After a median follow-up of 11 months (IQR 4.7–17.6), 16 patients died. Overall median PFS and OS were 6 months (95% CI 5–10) and 24 months (95% CI 13–24), respectively. Local progression occurred in 14 treated lesions (26.9%), 5 and 9 in the ablative and palliative RT group, respectively. Local control was significantly improved for lesions treated with ablative if compared to palliative intent (71.8 vs 37.5%, p = 0.0009). Distant progression occurred after treatment of 39 out of 52 lesions (75%), 19 and 20 in the ablative and palliative RT group, respectively. No difference in terms of distant control was detected for lesions treated with ablative if compared to palliative intent (32.1 vs 16.6%, p = 0.19). The absence of metastasis at diagnosis and ablative RT intent were significantly associated with improved PFS (9 vs 4 months, p = 0.005 and 20 vs 5 months, p < 0.0001, respectively). Metachronous metastases and concomitant ablative RT were also predictive of improved OS (not reached vs 11 months for both, p = 0.02 and p = 0.001, respectively). At multivariable Cox regression analysis, ablative RT intent was the only independent predictor of both PFS and OS (HR 3.51, 95% CI 1.6–7.5, p = 0.0012 and HR 2.8, 95% CI 0.99–8.07, p = 0.05, respectively) (Fig. 2). The overall toxicity profile of both SBRT and palliative RT was mild and principally related to ICI administration, with > G2 events occurring in 14 patients overall (7 endocrine, 2 skin rash, 1 pneumonitis, 3 hepatic, and 2 pancreatic).
Table 3.
Principal baseline features of patients included
| Characteristics | n (%) |
|---|---|
| RT intent | |
| Ablative | 19 (47.5) |
| Palliative | 21 (52.5) |
| Initial staging | |
| Synchronous metastases | 15 (37.5) |
| Metachronous metastases | 25 (62.5) |
| Metastatic site | |
| Bone | 22 (55) |
| Brain | 7 (17.5) |
| Visceral | 7 (17.5) |
| Nodal | 3 (7.5) |
| Soft tissues | 1 (2.5) |
| IMDC risk group | |
| Favorable | 15 (37.5) |
| Intermediate/poor | 25 (62.5) |
| Cytoreductive nephrectomya | |
| Yes | 14 (93.3) |
| No | 1 (6.7) |
| Treatment lines before Nivolumab | |
| 0 | 5 (12.5) |
| 1 | 25 (62.5) |
| ≥ 2 | 10 (25) |
aPercentages are related to patients with synchronous metastasis at diagnosis
Fig. 2.
Cox proportional hazard model for progression free and overall survival
Discussion
While limited by its retrospective nature, small sample size, relatively short follow-up, selection bias and confounding, our preliminary experience outlines the potential benefit of SBRT vs palliative RT for patients with both synchronous (after CN) and metachronous metastatic RCC treated with ICIs. In addition, it highlights the current unmet clinical need of exploring the indications and outcomes of multimodal treatment (i.e., ICI-based systemic therapy + / − CN + / − SBRT) in well-selected patients with metastatic RCC treated by multidisciplinary tumour boards.
In our study, ablative SBRT + ICI yielded significantly better oncologic outcomes in terms of PFS and OS and a higher degree of local control of metastatic lesions as compared to palliative RT. Of note, despite the promising impact on clinical history of these patients, no evidence of the so-called “abscopal effect” (increase in distant control in patients treated with SBRT) was noticed, raising concerns regarding its suitability as a key endpoint for a prospective clinical trial.
Our findings are consistent with an increasing body of evidence showing the potential added value of ablative RT in patients treated with ICI. In fact, in the RADVAX trial enrolling patients with RCC treated with ICI and concomitant SBRT, the overall response rate was 56% [9]. Similar results were found in the RAPPORT trial, enrolling patients treated with RT (either SBRT or conventional palliative RT if SBRT was not feasible) followed by pembrolizumab [10]; yet, data on the differential outcomes between ablative and palliative RT were not available.
Conclusion
In conclusion, our experience suggests that SBRT may improve local control and oncologic outcomes in carefully selected patients with metastatic RCC treated with ICIs as compared to palliative RT. In light of our study design, our findings are hypothesis-generating and should prompt the design of larger prospective clinical trials evaluating the added value of ablative RT in this setting.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
All authors have made a substantial contribution to research design, or the acquisition, analysis or interpretation of data. All authors have drafted the paper and revised it critically and have approved the submitted final version.
Ethics approval and informed consent
Ethical approval was waived by the local Ethics Committee of University of Florence in view of the retrospective nature of the study and all the procedures being performed were part of routine care. The study was performed according to the Declaration of Helsinki and written informed consent was obtained for all patients. Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/25/2022
Missing Open Access funding information has been added in the Funding Note.
References
- 1.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20(10):1370–1385. doi: 10.1016/S1470-2045(19)30413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563–1573. doi: 10.1016/S1470-2045(20)30436-8. [DOI] [PubMed] [Google Scholar]
- 3.Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ljungberg B, Albiges L, Bedke J, et al. EAU guidelines on renal cell carcinoma. 2021 edition. Available at: https://uroweb.org/guideline/renal-cell-carcinoma/.
- 6.Masini C, Iotti C, De Giorgi U, et al. Nivolumab (NIVO) in combination with stereotactic body radiotherapy (SBRT) in pretreated patients (pts) with metastatic renal cell carcinoma (mRCC): first results of phase II NIVES study. J Clin Oncol. 2020;38(6):613–613. doi: 10.1200/JCO.2020.38.6_suppl.613. [DOI] [Google Scholar]
- 7.Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18(4):240–243. doi: 10.1016/j.semradonc.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Hammers HJ, Vonmerveldt D, Ahn C, et al. Combination of dual immune checkpoint inhibition (ICI) with stereotactic radiation (SBRT) in metastatic renal cell carcinoma (mRCC) (RADVAX RCC) J Clin Oncol. 2020;38(6):614–614. doi: 10.1200/JCO.2020.38.6_suppl.614. [DOI] [Google Scholar]
- 9.Siva S, Bressel M, Wood S, et al. Stereotactic radiotherapy and pembrolizumab for oligometastatic renal tumors: the RAPPORT trial. J Clin Oncol. 2021;39(6):277–277. doi: 10.1200/JCO.2021.39.6_suppl.277. [DOI] [Google Scholar]