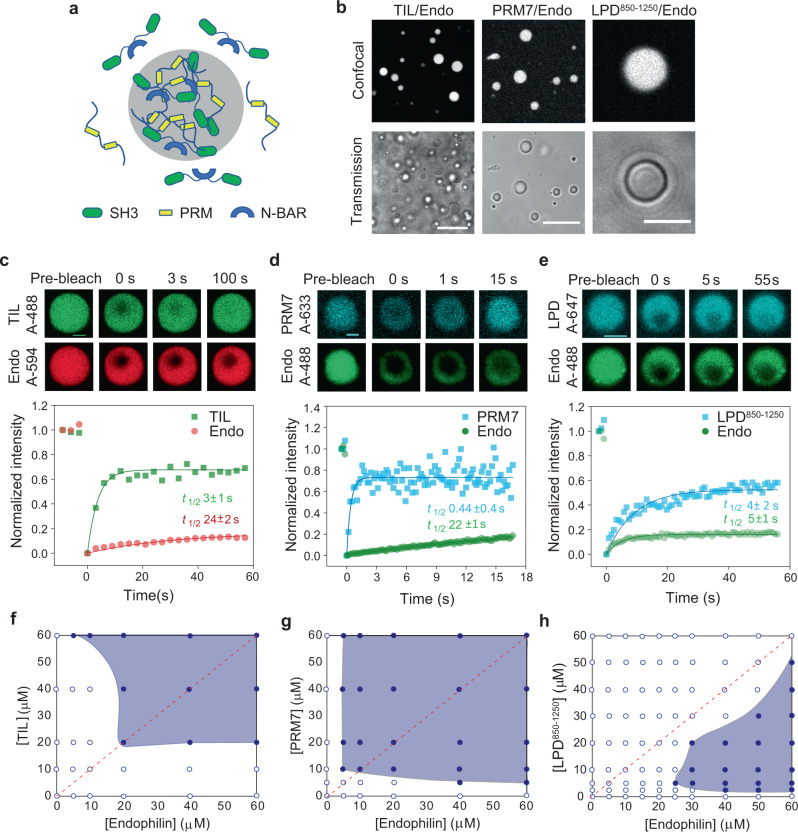
Fig. 3. Endophilin undergoes LLPS through multivalent interactions with proline-rich motifs in the absence of PEG.
a Cartoon diagram illustrating that multivalent interaction between SH3 domains of dimeric endophilin and a multiple PRM containing ligand can drive LLPS. b Confocal fluorescence images (top) and transmission images (bottom) of droplets formed by the TIL/endophilin, PRM7/endophilin, and LPD850–1250/endophilin system. The TIL/endophilin and PRM7/endophilin droplets were formed in the presence of 100 µM of endophilin and 100 µM of either TIL or PRM7 with 1 µM of either TIL-Alexa 488 or PRM7-Alexa 633. The LPD850–1250/endophilin droplets were formed by mixing 20 µM of LPD850–1250 and 60 µM of endophilin and contained 1 µM of LPD850–1250-Alexa 647. Scale bars 20 µm. c Confocal images and intensity profiles from a representative FRAP experiment on a TIL‒endophilin droplet to monitor the mobility of both endophilin (Alexa 594) and TIL (Alexa 488). Recovery time constants (t1/2) for TIL (green) and endophilin (red) were determined from single-exponential fits (solid lines) of the FRAP data and have been reported as mean±s.d. of three independent FRAP experiments. d, e FRAP studies on PRM7/endophilin and LPD850–1250/endophilin droplets in the presence of endophilin-Alexa 488, PRM7-Alexa 633, and LPD850–1250-Alexa 647. Recovery time constants (t1/2) for PRM7, LPD850–1250 (cyan), and endophilin (green) have been reported as mean±s.d. of three independent FRAP experiments. Scale bars 2 µm (c–e). f–h Phase diagrams for TIL/endophilin (f), PRM7/endophilin (g), and the LPD850–1250/endophilin (h) LLPS systems. The red dotted line represents the axis of a 1:1 mixing ratio of both proteins. All experiments were performed in 20 mM HEPES buffer, 150 mM NaCl, 1 mM TCEP, pH 7.4, and at room temperature.