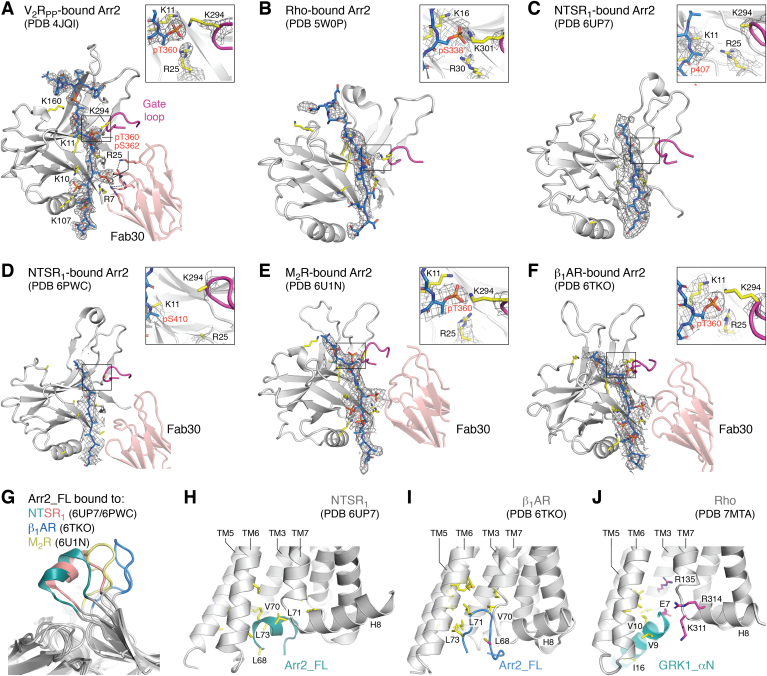
Figure 3.
Comparison of receptor-engaged phosphorylation and activation sensors.A–F, structures of arrestins bound to (presumably) phosphorylated C tails of GPCRs. When added, Fab30 interacts with the position analogous to pS362 in the V2Rpp peptide, effectively stapling arrestin to the GPCR C tail (D–F). Density for the GPCR C tail in each structure (gray wire cages) in general has poor definition, as evidenced by poor stereochemistry in some cases (e.g., see colliding adjacent phosphates in panel E). The insets detail the interactions at the most consistently observed phosphosite (corresponding to residue Thr360 in V2Rpp in panel A), which is coordinated by Arr2-Lys11, Arr2-Arg25, and presumably Arr2-Lys294 (because density is lacking). G, the Arr2 finger loop (76) shows many different conformations when bound to a GPCR, highlighting its ability to adapt to distinct cytoplasmic clefts and arrestin orientations relative to the receptor core. Shown is a superposition of Arr2 bound to β1AR (PDB entry 6TKO) (36), M2R (PDB entry 6U1N) (35), and NTSR1 (PDB entry 6PWC and 6UP7) (33, 34). H and I, interactions of Arr2 finger loop within the cytoplasmic cleft of (H) NSTR1 (PDB entry 6UP7) (34) and (I) β1AR (PDB entry 6TKO) (36). J, interaction of the GRK1 αN helix with the cytoplasmic cleft of Rho (PDB entry 7MTA) (52). In (H–J), the side chains of residues contributing hydrophobic and hydrophilic interactions are shown with yellow and magenta carbons, respectively. β1AR, β1 adrenergic receptor; Arr2, arrestin-2; GPCR, G protein-coupled receptor; GRK, G protein-coupled receptor kinase; H8, helix 8; M2R, M2 muscarinic receptor; NTSR1, neurotensin receptor 1; V2Rpp, vasopressin 2 receptor–derived phosphopeptide; Rho, rhodopsin; TM, transmembrane.