Figure 4.
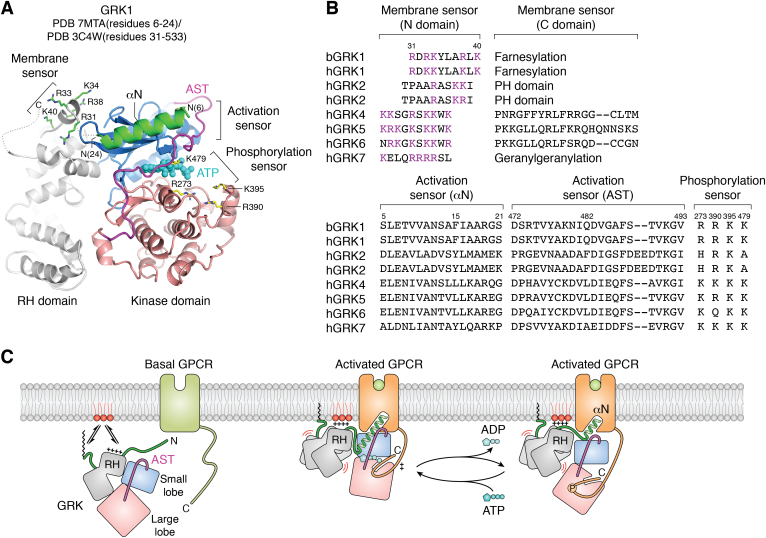
GRKs also contain conserved structural elements that serve as sensors for active, phosphorylated GPCRs and their surrounding anionic lipid environment.A, activated structure of GRK1. The model was generated by merging the GRK1 αN and kinase domain from the structure of GRK1 in complex with Rho (PDB entry 7MTA) (52) and the RH domain from the crystal structure of GRK1 in its basal state (PDB entry 3C4W) (77). The RH domain was in fact disordered in the Rho–GRK1 complex. ATP is modeled in place of the adenosine analog sangivamycin used in the Rho complex. The GRK “membrane” and “phosphorylation” sensors, by loose analogy to those of arrestin, are highlighted with yellow and green side chains, respectively. The activation sensor is composed of the N-terminal half of αN and adjacent segments of the AST loop (purple). B, sequence alignment of the GRK phosphorylation, activation, and membrane sensors. Residue numbering is based on bovine GRK1. The N domain membrane sensor mainly interacts with negative phospholipids via electrostatic interactions and participating residues are shown in purple. Note that a significant role in membrane binding for the residues in this region has not been experimentally demonstrated in GRK2 and 3. C, cartoon representation of GRK activation, membrane, and phosphorylation sensors in basal (left) and activated, GPCR-bound (center and right) states. The GRK is speculated to partially dissociate from the receptor during the exchange of ATP, remaining tethered to the receptor either via its activation or phosphorylation sensors. AST, active site tether; GPCR, G protein-coupled receptor; GRK, G protein-coupled receptor kinase; Rho, rhodopsin; RH, regulator of G protein signaling-homology.