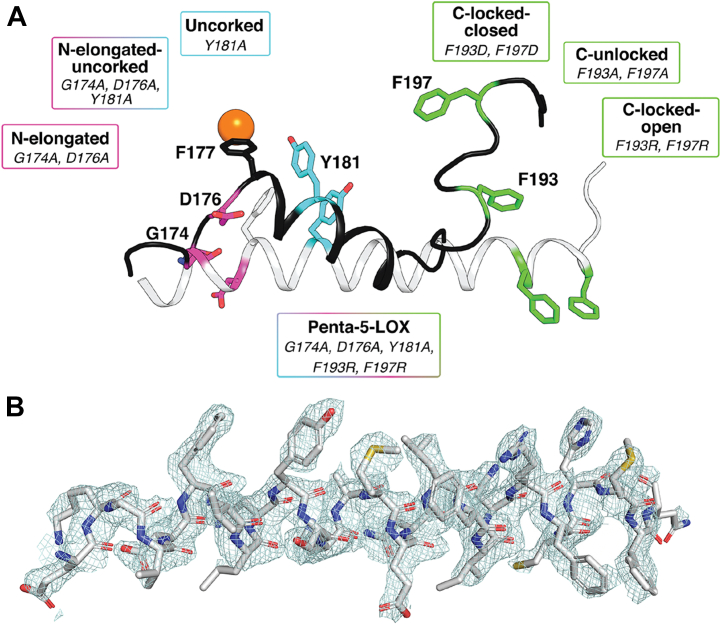
Figure 3.
Schematic overview of active and resting models of Stable-5-LOX created through mutagenesis and electron density of an elongated Hα2.A, Hα2 of 3O8Y.pdb is depicted in black as a cartoon with residues of interest shown as sticks. The alternate conformation of Hα2 is colored white. Pink colored sticks indicate amino-terminal mutated residues, light blue indicates the corking residue that was mutated, and green indicates carboxy-terminal residues that were mutated. F177 is shown as a stick. The active site iron is colored orange. B, electron density |2Fo –Fc| contoured to 1σ of the elongated version of Hα2 with residues depicted as sticks. Atoms are colored as follows: gray, carbon; red, oxygen; blue, nitrogen; yellow, sulfur. 5-LOX, 5-lipoxygenase; Hα2, helix-α2.