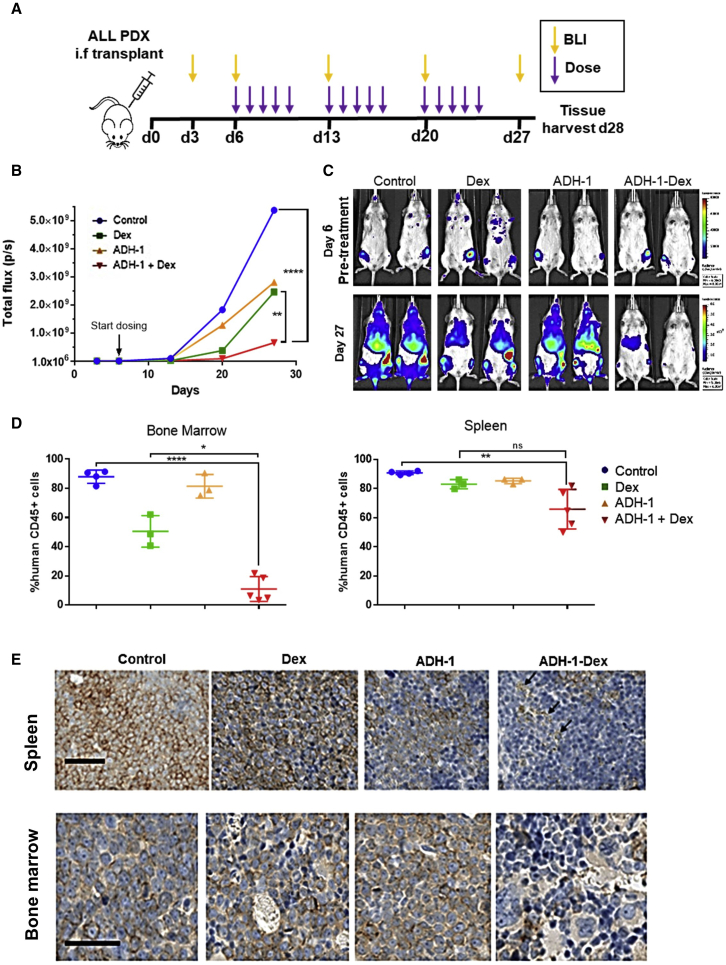
Figure 7.
ADH-1 potentiates dexamethasone sensitivity in vivo
(A) The PDX in vivo efficacy study design. Mice were dosed interperitoneally with either saline vehicle (control), 3 mg/kg dexamethasone (Dex), 200 mg/kg ADH-1, or ADH-1-Dex combined, 1× daily, 5× weekly for 3 weeks (15 doses), 5 mice per treatment group.
(B) Mean whole-body total flux measurements from bioluminescent imaging of each treatment group.
(C) Representative luminescence images of mice before and after treatment. Mice at each time point are shown with identical luminescence scale for comparison. Leukemic blasts are present in the femurs of all of the mice at the start of treatment. Signal spreads to BM sites, liver, and spleen in control mice, whereas signal is barely visible in ADH-1-Dex controls.
(D) Leukemic engraftment in harvested BM and spleen measured by flow cytometry of labeled harvested cells. Human CD45+ cells are shown as a percentage of total CD45+ cells (mouse + human cells). Lines indicate means and SEs, symbols for individual mice. ANOVA (GraphPad Prism), ns not significant, ∗p < 0.05, ∗∗p < 0.005, ∗∗∗∗p < 0.00005.
(E) Human CD19 immunohistochemistry on sections of spleen and bone harvested from mice. Mice treated with ADH-1-Dex combination have few CD19-stained cells (brown staining at the cell membranes) and have areas of punctate staining indicative of cell debris (arrows). Scale bar, 50 μm.