A list of chemotherapeutic nucleosidic analog prodrugs.
| Name | Structure | Statusa | Parent drug | Linker | Targeted cancer | |
|---|---|---|---|---|---|---|
| Capecitabine |
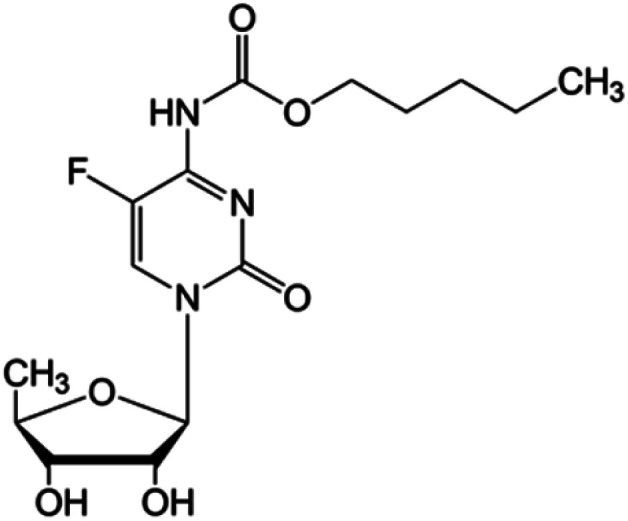
|
FDA approved (1998) | • Fluorouracil | • Amide bond | • Colonic neoplasms | 36 |
| EMA approved (2001) | • Breast neoplasms | |||||
| • Colorectal neoplasms | ||||||
| • Stomach neoplasms | ||||||
| Thymectacin |
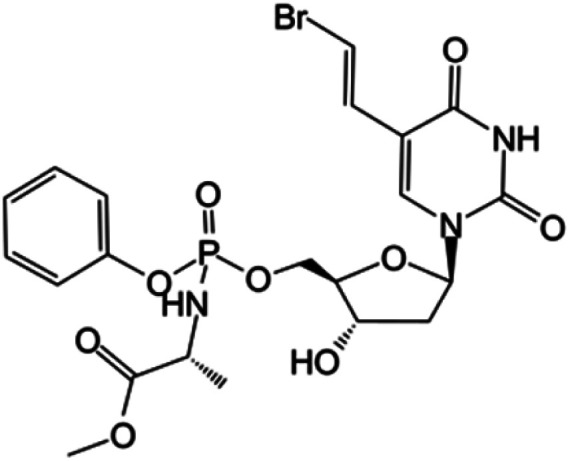
|
Clinical trials | • Brivudine | • Phosphate ester bond | • Cancers overexpressing the thymidylate synthase enzyme | 80 and 81 |
| Phase I/II | ||||||
| LY2334737 |
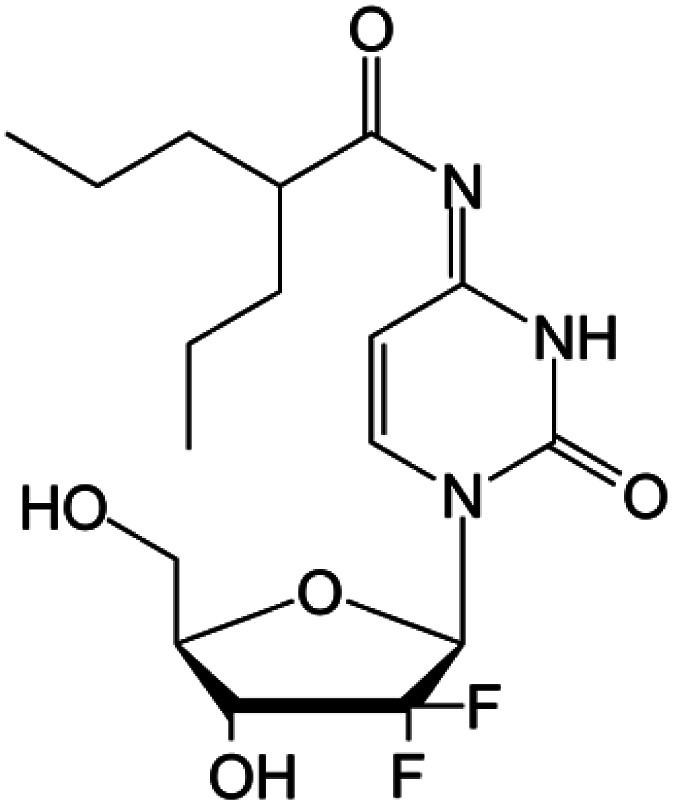
|
Clinical trials | • Gemcitabine | • Amide bond | • Malignant solid tumors | 82 |
| Phase I | • Metastatic tumors | |||||
| CP-4126 |
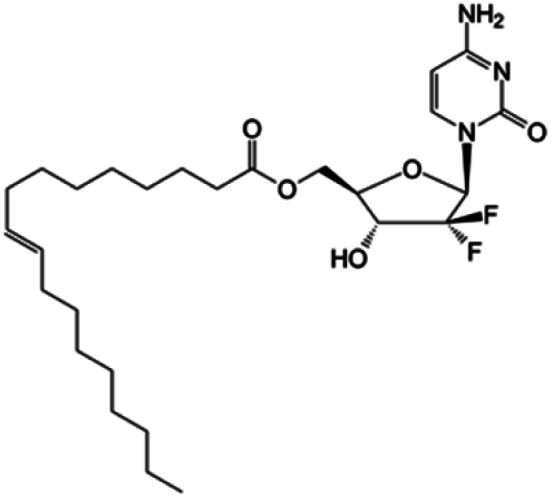
|
Clinical trials | • Gemcitabine | • Carboxyl ester bond | • Advanced adenocarcinoma of pancreas | 83 |
| Phase I/II | ||||||
| Nuc-1031 |
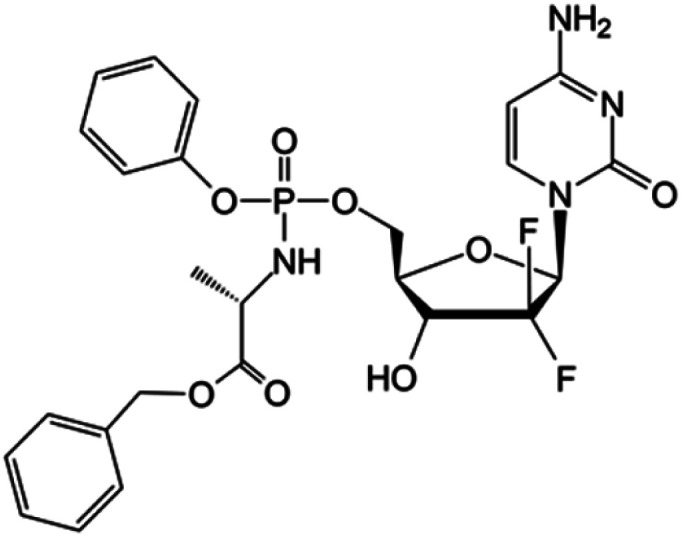
|
Clinical trials | • Gemcitabine | • Phosphate ester bond | • Ovarian cancer | 84 |
| Phase I/II/III | • Biliary tract cancer | |||||
| • Gallbladder cancer | ||||||
| • Cholangiocarcinoma | ||||||
| • Ampullary cancer | ||||||
| Sapacitabine |
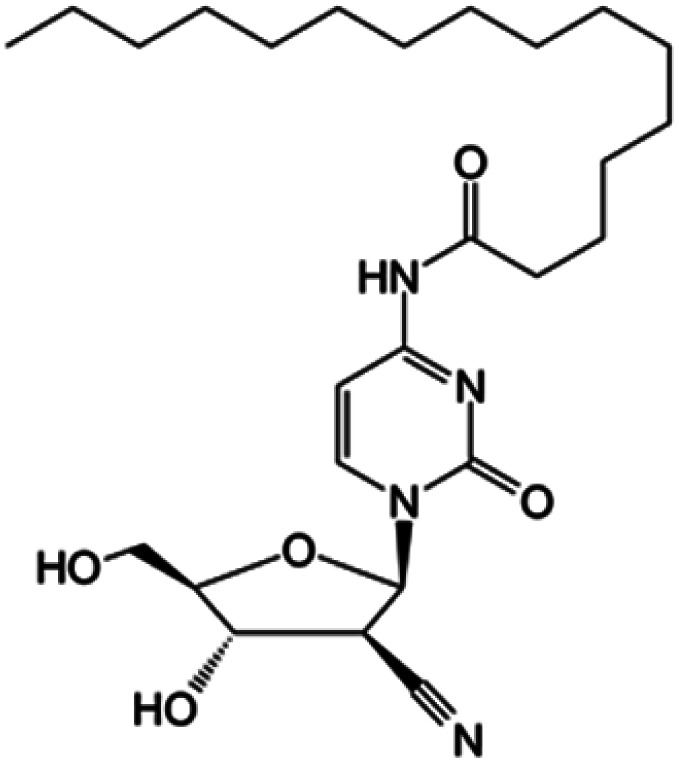
|
Clinical trials | • Cndac (deoxycytidine analog) | • Amide bond | • Myelodysplastic syndromes | 85 |
| Phase I/II/III | • Acute myeloid leukemia | |||||
| Guadecitabine (SGI-110) |

|
Clinical trials | • Decitabine | • Phosphate ester bond | • Small cell lung cancer | 79 and 86 |
| Phase I/II/III | • Ovarian cancer | |||||
| • Hepatocellular carcinoma | ||||||
| • Acute myeloid leukemia | ||||||
| ACV-TP-T |
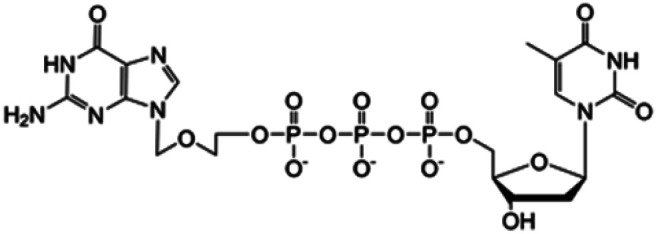
|
Preclinical | • Acylcovir | • Phosphodiester bond | • Pancreatic tumor | 87 |
Trial status was verified by the NIH – U.S. National Library of Medicine clinical trials database (http://www.clinicaltrials.gov). Approval status was verified by the European Medicines Agency (http://www.ema.europa.eu) and the US Food and Drug Administration (http://www.fda.gov).
