FIGURE 4.
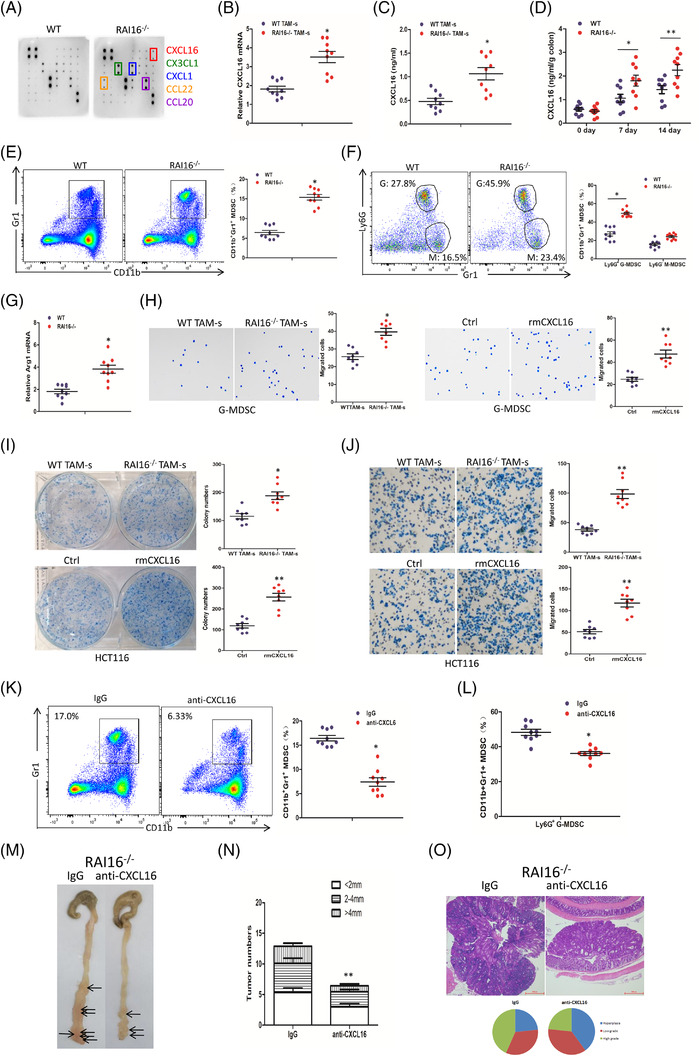
RAI16 deficiency increases CXCL16 production, which recruits immunosuppressive MDSCs and enhances tumour cell proliferation and migration. (A) Multi‐chemokine array proteome profiler (AAM‐CHE‐1, Raybiotech) of supernatants from primary TAMs derived from WT or RAI16–/– tumours. (B) Relative mRNA expression of CXCL16 in TAMs from tumours of WT or RAI16–/– mice was measured by qRT‐PCR. (C) The expression of CXCL16 by TAMs of WT or RAI16–/– mice was measured by ELISA. (D) The expression of CXCL16 in colon tissues WT or RAI16–/– mice with or without DSS treatment. (E) Gr1+CD11b+ MDSCs in tumour tissues of WT and RAI16–/– mice were assessed by flow cytometry. (F) Gr1+Ly6G+ G‐MDSCs and Gr1+Ly6G– M‐MDSC were further assessed from CD11b+ MDSCs by flow cytometry. (G) Lysates of distal colons from tumour‐bearing WT and RAI16–/– mice were prepared, and Arg1 mRNA expression was analysed using qRT‐PCR. (H) Transwell migration assay was performed in MDSCs by TAM culture supernatant (TAM‐s) or recombinant mouse CXCL16 (rmCXCL16, 10 ng/ml) treatment. (I, J) Colony formation assay (I) and Transwell migration assay (J) were performed in HCT116 cells by TAM‐s or rmCXCL16 (10 ng/ml) treatment. K‐M. Mice was intraperitoneally injected a neutralising CXCL16 antibody (anti‐CXCL16) or control antibody (IgG) once a week for 14 weeks. Gr1+CD11b+ MDSCs (K) or Gr1+Ly6G+ G‐MDSCs and Gr1+Ly6G– M‐MDSCs (L) in tumour tissues from anti‐CXCL16 and IgG‐treated RAI16–/– mice were assessed by flow cytometry. Tumour number (M), tumour size (N) and tumour grade (O) were analysed on day 100
