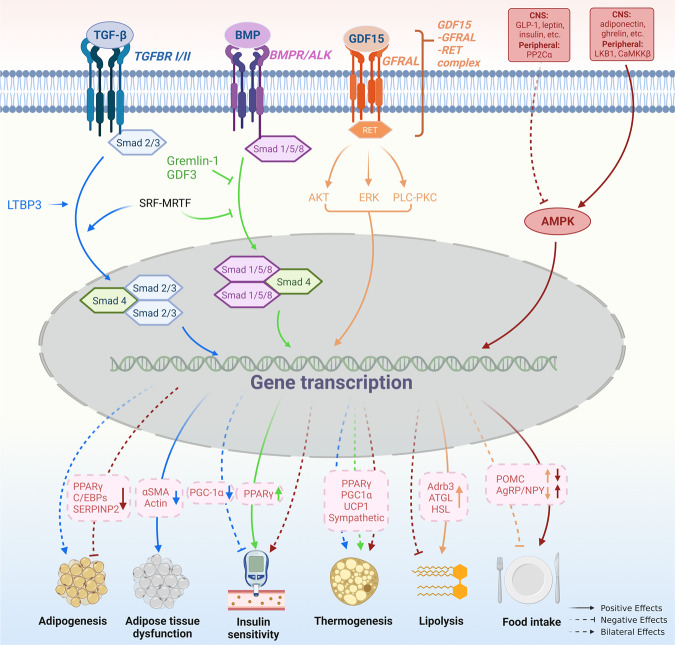
Fig. 3.
TGF-β and AMPK signaling pathways in obesity pathogenesis. The TGF-β superfamily consists of TGF-β1-3, GDFs, BMPs, etc., which play a diverse role in the development of obesity. TGF-β shows dual effects on adipogenesis/adipocyte differentiation. TGF-β inhibits MSC adipocyte commitment by phosphorylating and suppressing PPARγ and C/EBPs expression, through Smad3 signaling. However, pulsed TGF-β1 administration during the commitment phase shows a promotion effect on adipogenesis in MSC by down-regulating SERPINB2 expression. In adipocytes, TGF-β signaling is involved in adipose tissue dysfunction by enhancing the expression of myofibroblast signature genes. The role of TGF-β in BAT-associated thermogenesis is also controversial. Activation of TGF-β signaling by LTBP3 promotes WAT browning by modulating UCP1 expression, while hepatic TGF-β signaling contributes to HFD-induced steatosis and obesity by reducing mitochondrial respiration and inhibiting white-to-beige fat conversion. In addition, SRF - MRTF - axis which transcriptionally enhances the TGF-β but attenuates BMP signaling pathway suppresses brown adipogenesis. TGF-β/Smad3 signaling also plays a negative role in insulin sensitivity by suppressing PGC-1α expression in adipose tissue. BMP seems to play a contrary role to TGF- β in the regulation of insulin sensitivity by up-regulating PPARγ expression. Similar to TGF- β, the role of BMP in BAT-associated thermogenesis is inconsistent. BMP4 promotes WAT browning and this process is inhibited by Gremlin-1. However, BMP-4 signaling during the terminal differentiation phase can impair the acquisition of a mature brown adipocyte phenotype. GDF15, another member of TGF- β superfamily, was identified as a potential target for the treatment of obesity. By interacting with GFRAL and followed by the activation of AKT-, ERK-, and PLC-PKC signaling pathway, GDF15 stimulates lipolysis by up-regulating Adrb3, ATGL, and HSL expressions. It also inhibits food intake in a CNS-dependent manner via an unknown mechanism. AMPK is a heterotrimer complex. It is activated by adiponectin, ghrelin, etc. in CNS and LKB1 and CaMKKβ in peripheral tissue, and inactivated by GLP-1, leptin, etc. in CNS and PP2Cα in peripheral tissue. Activation of AMPK in CNS results in hyperphagia, insulin resistance, decreased thermogenesis, and weight gain. While, in adipocytes, it results in inhibited adipogenesis, insulin sensitiveness, enhanced thermogenesis, and weight loss. However, AMPK limits lipolysis since AMPK is an enzyme in case of energy shortage