Figure 3. One-pot synthesis of BCP-BF3K 6 and its diversification by photoredox-mediated processes.
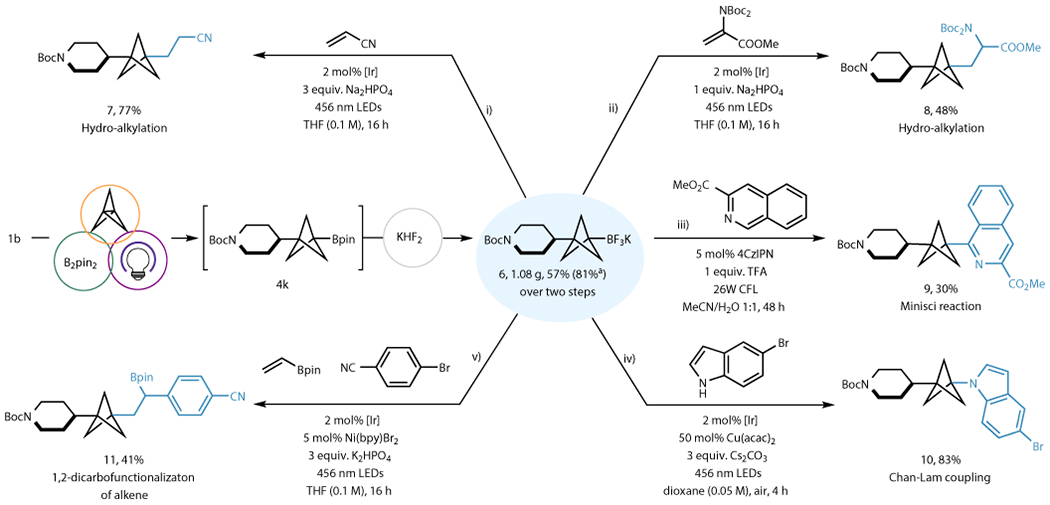
BCP trifluoroborate 6 can be synthesized in a one-pot manner from RAE without the need of column chromatography. The synthetic applications of 6 is demonstrated by several photoredox transformation: (i) hydroalkylation with acrylonitrile; (ii) hydroalkylation with dehydroalanine to yield an amino acid derivative; (iii) Minisci heteroarylation; (iv) single-electron mediated Chan-Lam C–N coupling; (v) 1,2-dicarbofunctionalization of vinyl boronic pinacol ester. See supplementary information section 7 for details on reaction conditions. [Ir]= Ir[dF(CF3)ppy]2(bpy)PF6. aYield by using isolated 4k.
