Figure 4. Mechanistic investigations into the radical intermediacy and origin of chemoselectivity.
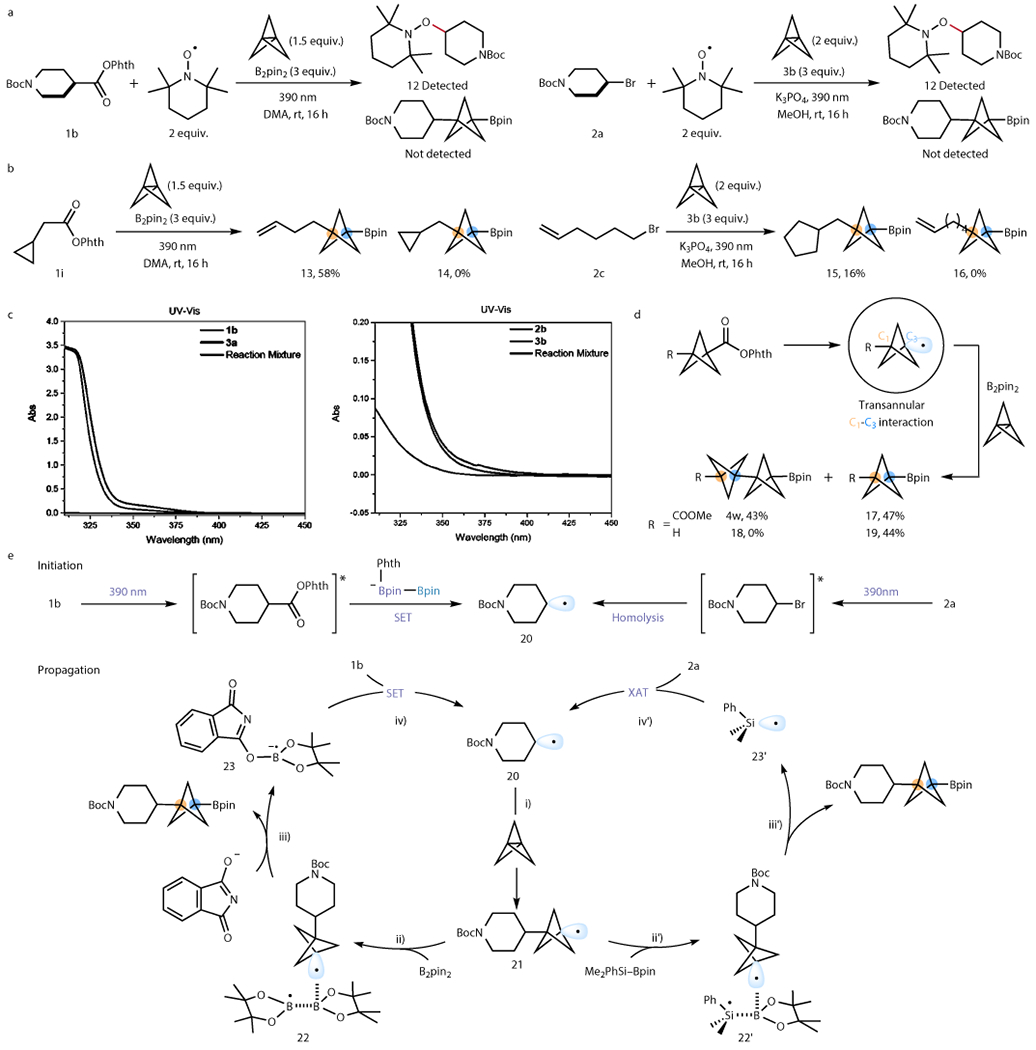
a. Addition of TEMPO completely shut down product formation, and TEMPO-adducts were formed. b. Radical clock experiments confirmed the radical nature of the two reactions and suggested slow addition between radical and [1.1.1]propellane. c. UV-vis studies indicate that at 390 nm, radical precursors are the only absorbing species, and there is no noticeable formation of an EDA complex. Thus, initiation is carried out by excitation of the RAE/organohalides. d. Electronic effect on BCP radical borylation vs. oligomerization: significant through-space effect on the BCP radical such that electron-rich BCP radical tends to undergo borylation whereas electron-poor BCP tends to oligomerize to form [2]staffane as the major product. e. Proposed mechanism based on literature and observations. The chain initiation involves light-mediated excitation of the radical precursor. Chain propagation involves four steps: i) radical addition to [1.1.1]propellane; ii) and ii’) the BCP radical formed coordinates to the Bpin acceptor; iii) and iii’) B–B/Si–B bond undergoes homolytic cleavage to generate BCP-Bpin; iv) and iv’) B/Si radical from the bond cleavage event initiates the next chain cycle.
