Table 1.
BCP Bpin and boronic acid synthesis by utilization of carboxylic acids as radical precursors
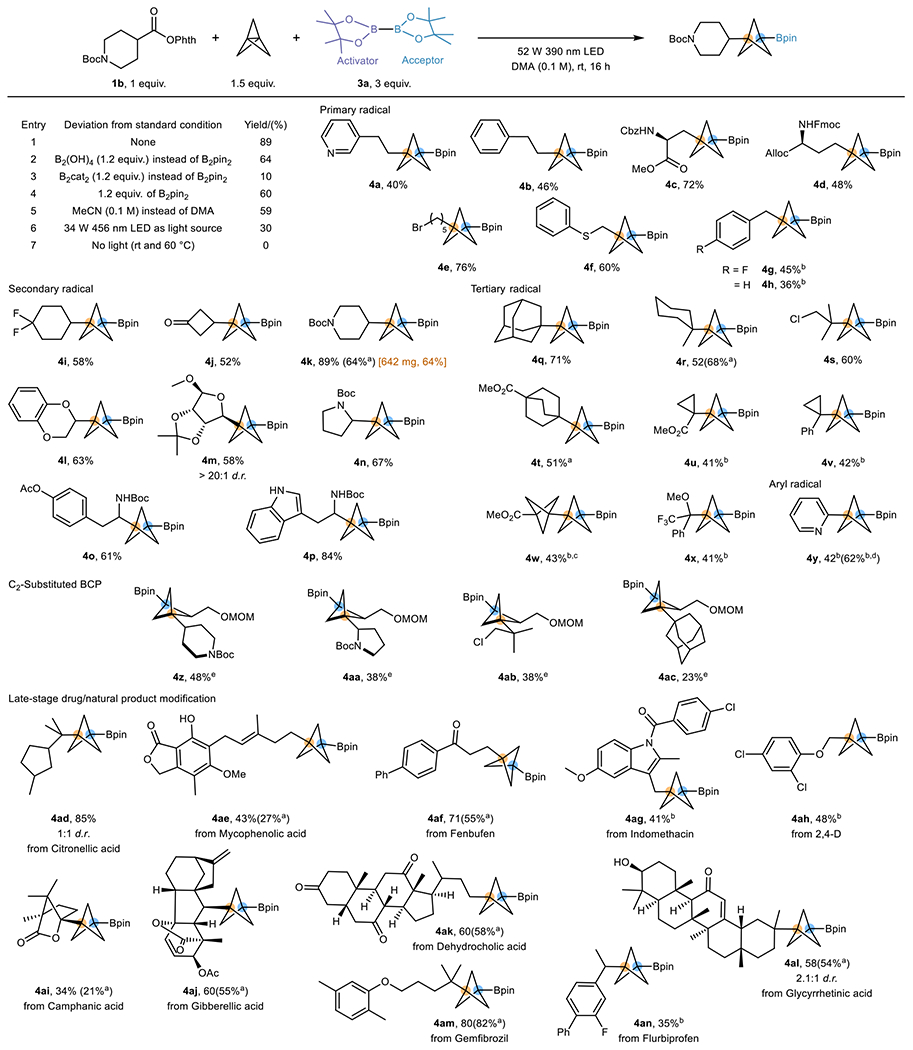
|
Optimization was performed on 0.3 mmol scale of 1b (See Supplementary Section 3.1 for details). Standard conditions for the scope study: RAE substrate (0.3 mmol, 1 equiv.), [1.1.1]propellane (0.45 mmol, 1.5 equiv.), B2pin2 (0.9 mmol, 3 equiv.), DMA (3 mL, 0.1 M), 16 h irradiation with 52 W 390 nm LEDs. All yields are isolated unless otherwise noted.
B2(OH)4 (1.2 equiv.) was used, and the yield was calculated by converting to the pinacolboronate by addition of pinacol (See Supplementary Section 4 for details).
3 equiv. of propellane were used.
1.2 equiv. of B2pin2 were used.
1H NMR yield using 1,3,5-trimethoxybenzene as internal standard
1.5 equiv. C2-substituted [1.1.1]propellane and 2 equiv. B2(OH)4 was used instead of [1.1.1]propellane and B2pin2. Products are racemic. (See Supplementary Section 4 for details).
