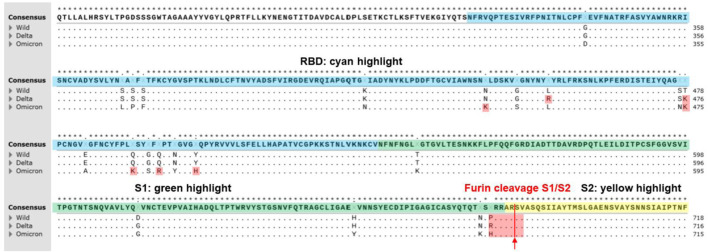
FIGURE 1.
A multiple sequence alignment of SARS-CoV2 S-proteins including Alpha, Delta, and Omicron variants reproduced from CLUSTALW. Conserved sequences showed the star symbol (*) on top of consensus sequence. Yellow highlight indicates N-terminal domain (13–304, NTD). Blue highlight indicates receptor binding domain (319–540, RBD). Green highlight indicates S1 subunit (541–683). Furin cleavage sites (S1/S2 sequence) at 684 (PRRAR↓SV) were highlighted on red. Positively charged mutations in RBD (L452R and T478K at Delta variant; N440K, T478K, Q493K, Q498R, and Y505H at Omicron variant) and furin cleavage sites (P681R at Delta variant; P681H at alpha and Omicron variant) were highlighted on red. Positively charged mutations (P681H and P681R) at furin cleavage sites of SARS-CoV-2 variants contributed more efficient cleavage (RRRAR↓SV > HRRAR↓SV > PRRAR↓SV) resulting in increased infectivity (Lubinski et al., 2021). Positively charged mutations in RBD may contribute tighter binding to the negatively charged ridges of ACE2 around the binding site of S-protein of SARS-CoV-2 (Prabakaran et al., 2004).