Abstract
Background
Economic evaluation of physical activity interventions has become an important area for policymaking considering the high costs attributable to physical inactivity. However, the evidence for such interventions targeting type 2 diabetes control is scarce. Therefore, the present study aimed to synthesize economic evaluation studies of physical activity interventions for type 2 diabetes management.
Methods
A systematic review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 statement (PROSPERO reference number CRD42021231021). An electronic search was performed in PubMed, Web of Science, Cochrane Library and NHS Economic Evaluation Database. Studies were eligible if they included: adults with type 2 diabetes; any physical activity intervention in the community settings; an experimental or quasi-experimental design; and a parameter of economic evaluation [cost analysis of interventions, cost-effectiveness analysis (including cost-utility analysis) and cost-benefit analysis] as an outcome.
Results
Ten studies were included in this review: seven were randomized controlled trials and three were quasi-experimental studies. All studies included direct costs, and four also included indirect costs. Four studies demonstrated that physical activity interventions were cost-saving, six studies showed cost-effectiveness, and two studies reported cost-utility. The estimates varied considerably across the studies with different analytical and methodological approaches.
Conclusion
Overall, this systematic review found that physical activity interventions are a worth investment for type 2 diabetes management. However, comparability across interventions was limited due to heterogeneity in interventions type, design and delivery, which may explain the differences in the economic measures.
Introduction
Diabetes is a major public health challenge of the century. In the last two decades, the prevalence of diabetes has increased alarmingly worldwide.1 In 2021, it was estimated that 537 million adults aged 20–79 years were diagnosed with diabetes.1 Obesity and physical inactivity have been associated with the increase in the prevalence of type 2 diabetes (T2D)—which accounts for around 90% of all diabetes cases worldwide.1
Physical activity is critical to the prevention and management of T2D.2,3 Studies have shown that physical activity decreases glycated hemoglobin, insulin resistance, fasting blood glucose, body mass index, body fat, blood lipids and blood pressure.4–6
Unfortunately, most people living with T2D remain physically inactive,7,8 and are, therefore, missing the opportunities to capitalize on the benefits, such as the reduced risk of cardiovascular events and overall mortality,9,10 and lowered healthcare costs. Recent studies showed that physical inactivity is costly and associated with a considerable disease burden.11 In 2013, physical inactivity was estimated to cost the healthcare system $53.8 billion globally, and, T2D to cost $37.6 billion to the healthcare system. Physical inactivity-related deaths also contributed to at least $13.7 billion in productivity losses and were responsible for 13.4 million disability-adjusted life-years worldwide.12
The economic evaluation of physical activity interventions plays an important role in informing policymaking and resource allocation, considering the constrained resources and competing priorities. In recent years, the economic evaluation of physical activity interventions has become an increasingly common practice. Evidence shows that some physical activity interventions are very cost-effective, such as various school-based interventions among children and adolescents, interventions using pedometers among adults, fall prevention programs among older people, and mass media campaigns and environmental approaches for the general population.13
Among people living with T2D, studies have found a varying degree of cost-effectiveness.14,15 Examples of interventions include intensive hypertension control in individuals with T2D (e.g. angiotensin-converting enzyme inhibitor therapy for intensive hypertension control compared with standard hypertension control); the use of pioglitazone plus metformin, a reportedly cost-saving therapy when compared with rosiglitazone plus metformin; comprehensive foot care to prevent ulcers compared with the usual care; counseling and treatment for smoking cessation compared with no counseling and treatment; intensive glycemic control in persons with newly diagnosed T2D compared with conventional glycemic control.14,15 However, evidence regarding the economic evaluation of physical activity interventions for T2D management remains scarce.
Therefore, we aim to systematically review economic evaluation studies of physical activity interventions for T2D management. Specifically, we aimed to summarize cost analysis of interventions (CAI), cost-effectiveness analysis (CEA) (including cost-utility analyses [CUA]), and cost-benefit analysis (CBA) of physical activity interventions in T2D.
By synthesizing and critically appraising existing economic evaluation studies of physical activity interventions in T2D, this study intends to assist decision makers with resource allocation and intervention selection.16
Methods
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement17 (Supplementary file S1). The protocol of this systematic review was registered in PROSPERO—reference number CRD42021231021.
Eligibility criteria
Studies were eligible if participants were adults diagnosed with T2D; included any physical activity intervention in the community settings; included experimental and quasi-experimental studies; outcome measures included a parameter of economic evaluation—CAI, CEA (including CUA) or CBA.
We excluded studies with multicomponent interventions (e.g. combined physical activity and diet), and studies with multimorbidity including T2D but without separate data for T2D.
Information sources
Electronic searches were conducted in PubMed, Web of Science, Cochrane Library and NHS Economic Evaluation Database.
Search strategy
For database search, we used the following keywords: (‘physical activity’ OR exercise OR ‘active transport’ OR ‘active mobility’ OR ‘active commuting’ OR ‘active travel’ OR walking OR cycling OR running OR training OR sport*) AND (diabet* OR ‘glycemic control’ OR ‘glycaemic control’ OR ‘glucose control’) AND (cost* OR cost-effectiveness OR cost-utility OR cost-benefit OR ‘economic evaluation’ OR ‘economic analysis’ OR ‘economic assessment’ OR ‘economic impact’). We did not apply language and date limits to the searches.
Detailed search strategy is available in Supplementary file S2.
Selection process
Two authors (A.B. and R.M.) independently reviewed the search results and screened records retrieved from databases according to predefined steps. First, records were screened based on the information from the title and abstract. Second, potentially relevant articles were retrieved for full-text reading and to determine their eligibility. In case of disagreement, the consensus was reached through discussion.
Data collection process
Two authors (A.B. and R.M.) independently extracted data from eligible studies. Then, retrieved data were compared and discussed if discrepancies existed. A third author (S.W.) reviewed entered data for accuracy. In case of unclear information, the original studies’ authors were contacted to provide additional clarification.
Data items
We considered studies eligible if they presented at least one of the following economic evaluation outcomes:
Cost analysis of interventions (CAI): estimation of the costs of an intervention’s implementation. It can include direct costs (costs of resources used to design and implement an intervention, such as personnel time, facility rent, supplies, and medications), productivity losses (impacts of patient participation in an intervention, such as work time lost or leisure-time lost due to participation in the intervention) and intangible costs (non-financial costs, such as pain and suffering, which impose a major burden on individuals).18
Cost-effectiveness analysis (CEA): comparison between the costs and the effectiveness of two or more interventions with effectiveness measured in the same units. The incremental cost-effectiveness ratio (ICER) is used to compare interventions—the difference in costs divided by the difference in health effects. Health effects are frequently measured through quality-adjusted life-years (QALYs) gained, disability-adjusted life-years (DALYs) averted, reduction of glycated hemoglobin, increase in daily steps, reduction in body fat, etc.19 Generally, when the effectiveness is measured through QALYs, the term cost-utility analysis (CUA) can be used.15
Cost-benefit analysis (CBA): comparison of the costs (including those of implementing an intervention) and benefits (including those resulting from an intervention, such as medical costs averted, productivity gains and the monetized value of health improvements) of an intervention. The unity of analysis is monetary.18
For each study, we summarized the following characteristics: first author, year of publication, country, design, intervention and comparison groups (type, duration, measurement, mode of delivery), study length, setting, condition (e.g. T2D vs multimorbidity of T2D), sample size (including sample size for each group, if available), and participants’ age and sex. In addition, we extracted the following methodological information from the studies: the perspective of the analysis, type of economic evaluation, cost analysis, and health outcomes. Finally, we extracted the key findings and the authors’ interpretation of the economic evaluation.
Study quality assessment
The assessment of the reporting quality of economic evaluation studies was performed by two independent authors (A.B. and R.M.), using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement.20 This statement consists of a 24-item checklist subdivided into six main categories: (i) title and abstract; (ii) introduction; (iii) methods; (iv) results; (v) discussion; and (vi) other.
Effect measures
CAI usually considers the total cost of the interventions’ implementation.
For CBA, the expected monetary benefits of the intervention are subtracted from the total cost of the interventions’ implementation.
For CEA (including CUA), interventions can be classified as cost-saving (an intervention that generates a similar health outcome with fewer costs than the comparison intervention) or cost-effective, according to the interpretation of the intervention impact in the variable that is used to measure the effectiveness (e.g. reduction in glycated hemoglobin, increase in daily steps, reduction in body fat, reduction in medications prescription). ICER—the difference in costs divided by the difference in health effects—is commonly used to measure cost-effectiveness and cost-utility, and it can be compared with thresholds based on per capita national incomes, benchmark interventions, or league tables.19
Synthesis methods
We conducted a narrative synthesis of included studies.
For the comparison of national estimates from different years and in different currencies, we converted all to purchasing power parity (PPP) international dollars using conversion factors provided by the World Bank,21 and considering the cost estimate year that studies provided. If a study did not mention the year used in cost analysis, we assumed the cost was the year of publication.
We did not perform meta-analysis to synthesize the results since we found many sources of heterogeneity across the studies.22
Results
Study selection
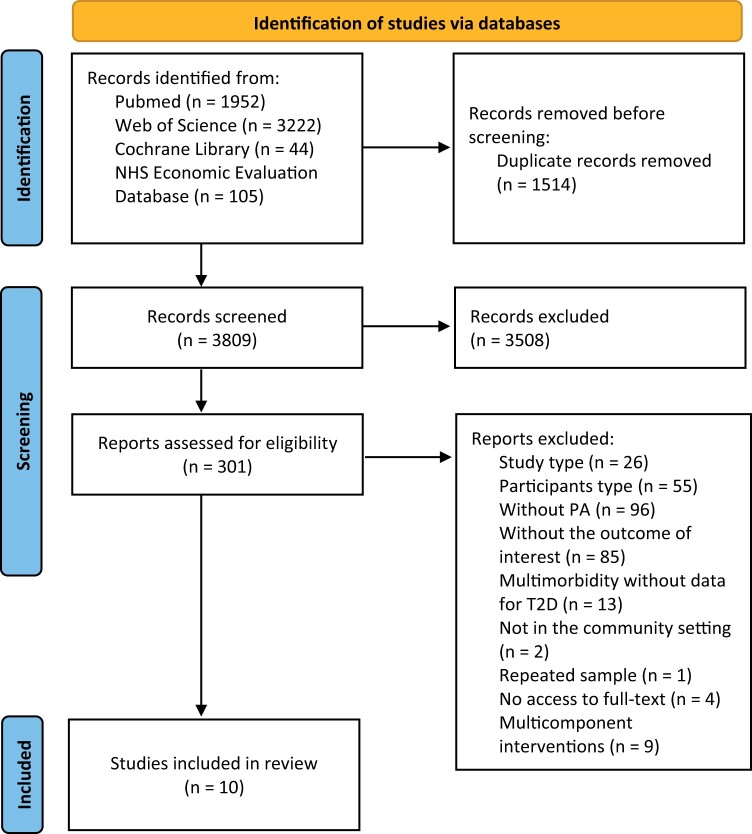
A total of 5323 references were identified in the initial search in electronic databases. After the duplicated studies (n = 1514) were removed, 3809 studies remained. After screening for the title and abstract, 3508 papers were excluded, and 301 studies were eligible for full-text reading, from which 291 were removed. Thus, the selection process resulted in the inclusion of 10 studies in the qualitative synthesis (figure 1).
Figure 1.
PRISMA 2020 flow diagram. PA, physical activity; T2D, type 2 diabetes
Study characteristics
The study characteristics included in this review are shown in table 1.
Table 1.
Characteristics of included studies
| Author, year | Country | Study design | Intervention group | Comparison group | Length | Setting | Condition | Sample (n) | Age range (mean ± SD), years | Sex |
|---|---|---|---|---|---|---|---|---|---|---|
| Brun et al., 200828 | France | RCT | Exercise program (including brisk walking, jogging or gymnastics) | Usual care | 12 months | Community | T2D | 25 (13 IG, 12 CG) | 40–85 (59.7 ± 2) | M + F(26.0% F) |
| Coyle et al., 201226 | Canada | RCT | Aerobic exercise; resistance exercise; combined exercise | Usual care | 6 months | Community | T2D | 251 | 39–70 (54.2) | M + F(34.9% F) |
| Di Loreto et al., 200532 | Italy | Quasi-experimental | Exercise counseling + phone calls + sessions in outpatient clinic | None | 24 months | Community | T2D | 179 | >40 (62 ± 1) | M + F |
| Johnson et al., 201527 | Canada | Quasi-experimental | Pedometer-based walking program | Usual care with a pedometer but without instructions | 6 months | Primary Health Care | T2D | 186 (94 IG, 92 CG) | ≥18 (59.3 ± 8.3) | M + F(50.0% F) |
| Kaplan 198823 | USA | RCT | Exercise; diet; diet + exercise | Education | 18 months | Community | T2D | 76 | 53.8 ± 8.0 exercise; 54.9 ± 12.3 diet; 56.9 ± 8.9 diet + exercise; 54.5 ± 8.8 CG | M + F(57.9% F) |
| Kuo et al., 202124 | USA | RCT | Exercise program (EXER); CBT; combined exercise program (EXER) + CBT | Usual care | 15 months | Community | T2D with major depressive disorder | 140 (EXER 34, CBT 36, EXER + CBT 34, CG 36) | 56.0 ± 10.7 | M + F(76.0% F) |
| Marios et al., 201229 | Australia | RCT | Walking exercise program monitored by heart rate monitors + phone calls | Walking program | 6 months | Community | T2D | 26 (15 IG, 11 CG) | 18–80 (60.3 ± 9.4 IG, 65.1 ± 7.9 CG) | M + F(33.0% F in IG, 64.0% F in CG) |
| Pepin et al., 202025 | USA | Quasi-experimental | Aerobic + resistance + balance exercise program | None | 12 months | Community | Multimorbidity | 453 | 31–91 (67 ± 10) | M + F(6.0% F) |
| Sultana et al., 201830 | Malaysia | RCT | Aerobic exercise; combined (aerobic + resistance/strengthening) exercise program | Usual care | 14 weeks | Community; hospital | T2D | 75 (25 aerobic training, 25 combined, 25 CG) | 40–60 | M + F |
| Taylor et al., 202031 | UK | RCT | Exercise referral schemes + e-coachER (a pedometer + fridge magnet with PA recording sheets, and a user guide to access the web-based support in the form of seven ‘steps to health’) | Exercise referral schemes alone | 12 months | Community | Multimorbidity | 450 (224 IG, 226 CG) | 50 ± 13 IG, 51 ± 14 CG | M + F(64.0% F) |
CBT, cognitive behavioral therapy; CG, control group; F, female; IG, intervention group; M, male; RCT, randomized controlled trial; SD, standard deviation; T2D, type 2 diabetes.
All studies were published in English, between 1988 and 2021. Half of the studies were conducted in the USA23–25 and Canada.26,27 Seven studies were randomized controlled trials (RCT)23,24,26,28–31 and three were quasi-experimental studies.25,27,32 All studies included interventions conducted in the community settings, although one study also included a hospital setting.30 The length for included studies varied from 14 weeks to 24 months.
All studies included participants with T2D. Eight studies included only patients with T2D,23,24,26–30,32 and two studies had individuals with other chronic conditions but provided data for T2D.25,31 The participants’ age ranged from 18 to 91, and most participants were middle-aged.
Regarding interventions, three were walking programs,27,29,30 and eight were multicomponent exercise programs (e.g. aerobic, resistance, combined aerobic and resistance exercise programs, etc.).23–26,28,30–32 Two interventions additionally included phone calls for monitoring weekly minutes of physical activity and counseling.29,32 Two interventions included pedometers27,31 and one intervention included a heart rate monitor29 for physical activity self-monitoring.
Most of the comparator groups included participants who followed usual care,24,26–28,30,31 one study had education,23 one included a walking program only29 and one received a pedometer only.27
Study quality assessment in studies
The assessment of methodological quality for each study is presented in Supplementary file S3.
Most of the studies adhered to the CHEERS checklist in the categories of title and abstract, introduction, discussion and other. All the studies did not comply with methods and results’ items, especially the choice of model, assumptions and uncertainty characterization.
Results of individual studies and synthesis
Results of individual studies are presented in table 2. Four studies took the sole perspective of healthcare,23,25,28,29 four studies combined healthcare and societal perspectives24,26,31,32 and two studies the payer perspective.27,30
Table 2.
Results of individual studies
| Study | Perspective | Economic evaluation | Cost analysis | Health outcomes | Findings | Authors’ interpretation of the economic evaluation |
|---|---|---|---|---|---|---|
| Brun et al., 200828 | Healthcare | CEA | Costs
|
Body composition, fitness, metabolic balance, diabetes treatment. |
|
Intervention is cost-saving. |
| Coyle et al., 201226 | Healthcare; societal |
|
Costs:
|
Life expectancy and quality-adjusted life expectancy. |
|
The combined exercise program is more cost-effective than aerobic, resistance or no exercise program in the improvement of T2D control |
| Di Loreto et al., 200532 | Healthcare; societal | CEA | Costs
|
Improvement in the 10-year coronary heart disease risk, glycemic control and cardiovascular risk factors, reduction in medical and social costs. |
|
Intervention is cost-saving. |
| Johnson et al., 201527 | Payer |
|
Costs
|
Change in daily steps. |
|
Intervention is cost-effective in increasing number of daily steps. |
| Kaplan, 198823 | Healthcare |
|
Costs
|
Quality of well-being. |
|
The diet and exercise groups have cost-utility, compared with other behavioral programs. |
| Kuo et al., 202124 | Healthcare; societal |
|
Costs
|
Incidence of clinical outcomes (e.g. stroke, cardiovascular death, myocardial infarction), life expectancy and quality-adjusted life expectancy. |
|
Exercise program and exercise program + CBT interventions are cost-saving; exercise program + CBT is more cost-effective than exercise program or CBT alone. |
| Marios et al., 201229 | Healthcare | CAI CEA |
Costs
|
Exercise adherence (number of hours of exercise completed), improvements in peak maximal oxygen uptake, glycated hemoglobin and quality of life; cost-effectiveness of exercise training compared with pharmacological therapy. |
|
The amount invested in intervention is comparable with other health interventions and it improved some health outcomes. |
| Pepin et al., 202025 | Healthcare | CEA | Costs
|
Changes in medication use and cost of medication classes commonly prescribed in the management of chronic conditions. |
|
Intervention is cost-saving. |
| Sultana et al., 201830 | Payer |
|
Costs
|
Glycated hemoglobin and health status. |
|
Combined exercise program is more cost-effective than aerobic exercise or CG. |
| Taylor et al., 202031 | Healthcare; societal |
|
Costs (direct and indirect):
|
Quality of life, and minutes of moderate and vigorous PA in ≥10-min bouts. |
|
|
CAI, cost analysis of interventions; CBT, cognitive behavioral therapy; CEA, cost-effectiveness analysis; CG, comparator group; CUA, cost-utility analysis; IG, intervention group; MET, metabolic equivalent of task; PA, physical activity; QALYs, quality-adjusted life years; T2D, type 2 diabetes.
All studies included estimates of direct healthcare costs of physical activity interventions. Direct costs included medical costs (outpatient care, laboratory testing, expenses with medications, hospitalizations and emergency care). Four studies also provided indirect costs,24,28,31,32 and included periods of not working, job loss and unemployment, and participants’ time spent in physical activity sessions.
Regarding economic evaluation, nine studies included CEA,24–32 and two studies used CUA.23,31 No CBA was identified in this review.
Physical activity interventions were reported as being cost-saving in four studies,24,25,28,32 six were considered cost-effective24,26,27,29–31 and two with cost-utility (table 3).23,31
Table 3.
Summary of result of the economic evaluation in individual studies
| Study | Result of the economic evaluation |
|---|---|
| Brun et al., 200828 | Cost-saving |
| Coyle et al., 201226 | Cost-effectiveness |
| Di Loreto et al., 200532 | Cost-saving |
| Johnson et al., 201527 | Cost-effectiveness |
| Kaplan, 198823 | Cost-utility |
| Kuo et al., 202124 | Cost-saving |
| Cost-effectiveness | |
| Marios et al., 201229 | Cost-effectiveness |
| Pepin et al., 202025 | Cost-saving |
| Sultana et al., 201830 | Cost-effectiveness |
| Taylor et al., 202031 | Cost-utility |
| Cost-effectiveness |
Discussion
This systematic review summarized the available evidence on economic evaluation studies of physical activity interventions in the context of T2D control. Of the 10 studies included, nine were conducted in high-income countries, and only one was in a middle-income country (Malaysia).30 Overall, we found that physical activity interventions are a worth investment for T2D control. Four interventions were considered cost-saving,24,25,28,32 six were considered cost-effective24,26,27,29–31 and two were considered to have cost-utility.23,31
In the literature, we can find other examples of interventions that are cost-effective in T2D management. Examples include interventions for hypertension control (angiotensin-converting enzyme inhibitor therapy for intensive compared with standard hypertension control); multicomponent interventions for diabetic risk factors control and early detection of complications, compared with standard glycemic control for persons with T2D; intensive lifestyle interventions to prevent T2D among persons with impaired glucose tolerance, compared with standard lifestyle recommendations; annual screening for diabetic retinopathy and ensuing treatment in persons with T2D, compared with no screening.14,15
Despite the encouraging findings on the investment of physical activity interventions for T2D control highlighted in the current review, it should be noted that only four studies used glycated hemoglobin as the endpoint of T2D control.28–30,32 Other studies used endpoints such as a change in daily steps27 or a change in exercise volume29,31 that can indirectly produce health benefits in people with T2D. However, these changes may not be sufficient to improve glycated hemoglobin, as noted by Marios et al.29
Consistent in this review, combined aerobic and resistance exercise programs showed cost-effectiveness when compared with the usual care.26,30 These results align with the physical activity guidelines for individuals with T2D, which recommend the inclusion of aerobic, resistance, flexibility and balance exercise training.2,3 These findings also encourage decision makers to allocate resources to multi-modal physical activity interventions.
The reported costs of three interventions were around $1000 per participant,23,27,29 which is a promising finding since it is similar to the annual per capita expenditure ($1423 in 2012 US dollars) on prescription medications for persons with T2D in the USA.33 Additionally, costs are likely to decrease over time with, for example, the reduction of the prescribed medication for T2D and cardiovascular risk factors.25,32 Moreover, regular practice of physical activity contributes to preventing and managing cognitive and mental health conditions and improving physical function across the lifespan.34,35
In this review, most studies took a healthcare perspective.23–26,28,29,31,32 Despite being very relevant for policymakers, upcoming studies should consider the societal perspective, which incorporates the economic impact on society, including the health sector (direct costs to public and private healthcare systems), non-health sector (indirect costs or productivity losses) and households (impact on usual activities).36 By taking a societal perspective, the economic evaluation will be relevant to stakeholders from different sectors, considering that physical activity interventions may involve non-health sectors and the costs of physical inactivity are borne by different sectors of the society.
This study has several limitations that need to be addressed. First, high heterogeneity in interventions type, design and delivery was noted in the included studies, which may explain the differences in the economic measures. Second, similar to a previous systematic review of physical activity-related cost-of-illness analysis, important information, such as time horizon, assumptions, sensitivity analyses, discounting, uncertainty, economic perspective, physical activity and economic measures calculation, was often not reported.11 Incomplete reporting has further complicated comparison between studies. Reporting of future studies should follow the best-practice standards for economic evaluation studies, such as the CHEERS statement.20 Moreover, recruitment costs were not considered in the majority of the studies, which may have resulted in the underestimation of intervention costs, which is an important point of consideration if programs are scaled up. Third, we did not include multicomponent interventions (such as diet plus exercise), which seem to be more effective and cost-effective for T2D control.37
Future research should involve larger studies with robust designs to establish the effects of physical activity interventions on T2D management and its cost-effectiveness, increase the reliability of findings and, ultimately, promote their use by policymakers.
It would also be important to investigate the impact of physical activity interventions on long-term outcomes related to T2D, namely in the incidence of cardiovascular diseases, micro- and macrovascular complications, years of life lost, and mortality. Furthermore, it was possible to note that long-term follow-up studies tend to be more cost-effective given that health benefits often last beyond the study period.
Conclusion
In conclusion, this systematic review found that physical activity interventions are a worth investment for type 2 diabetes management. However, due to the studies’ heterogeneity, it is challenging to compare interventions across studies.
Studies with a societal perspective and robust analysis over wider time horizons are needed to explore the potential of physical activity interventions in the effectiveness and cost-effectiveness of T2D management over the long term. This will allow for efficient resource allocation by policymakers across the sectors involved in implementation programs.
Supplementary data
Supplementary data are available at EURPUB online.
Funding
This work was funded by the Portuguese Foundation for Science and Technology within the scope of projects UIDB/04750/2020 and LA/P/0064/2020. Ana Barbosa was supported by the Portuguese Foundation for Science and Technology, grant number SFRH/BD/136702/2018.
Disclaimer
S.W. is a WHO staff member and R.M. is a WHO consultant. The authors alone are responsible for the views expressed in this publication. They do not necessarily represent the views, decisions, or policies of the institutions they are affiliated with.
Conflicts of interest: none declared.
Key points.
Physical activity interventions are a good investment for type 2 diabetes management.
Health benefits of physical activity interventions last beyond the intervention period.
Future studies should include a societal perspective, robust design and accurate reporting to better inform resource allocation and decision making.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
Contributor Information
Ana Barbosa, EPIUnit—Instituto de Saúde Pública, Universidade do Porto, Porto, Portugal; Laboratório para a Investigação Integrativa e Translacional em Saúde Populacional (ITR), Porto, Portugal.
Stephen Whiting, EPIUnit—Instituto de Saúde Pública, Universidade do Porto, Porto, Portugal; Laboratório para a Investigação Integrativa e Translacional em Saúde Populacional (ITR), Porto, Portugal; World Health Organization, Regional Office for Europe, Copenhagen, Denmark.
Ding Ding, Prevention Research Collaboration, Sydney School of Public Health, The University of Sydney, Camperdown, Australia.
João Brito, Portugal Football School, Portuguese Football Federation, Oeiras, Portugal.
Romeu Mendes, EPIUnit—Instituto de Saúde Pública, Universidade do Porto, Porto, Portugal; Laboratório para a Investigação Integrativa e Translacional em Saúde Populacional (ITR), Porto, Portugal; World Health Organization, Regional Office for Europe, Copenhagen, Denmark; Portugal Football School, Portuguese Football Federation, Oeiras, Portugal; ACES Douro I—Marão e Douro Norte, Northern Region Health Administration, Vila Real, Portugal.
References
- 1. International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels, 2021. Available at: https://diabetesatlas.org/atlas/tenth-edition/ (10 February 2022, date last accessed). [PubMed] [Google Scholar]
- 2. American Diabetes Association Professional Practice Committee. 5. Facilitating behavior change and well-being to improve health outcomes: standards of medical care in diabetes—2022. Diabetes Care 2021;45:S60–82. [DOI] [PubMed] [Google Scholar]
- 3. Kanaley JA, Colberg SR, Corcoran MH, et al. Exercise/physical activity in individuals with type 2 diabetes: a consensus statement from the American College of Sports Medicine. Med Sci Sports Exerc 2022;54:353–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sampath Kumar A, Maiya AG, Shastry BA, et al. Exercise and insulin resistance in type 2 diabetes mellitus: a systematic review and meta-analysis. Ann Phys Rehabil Med 2019;62:98–103. [DOI] [PubMed] [Google Scholar]
- 5. Zhao X, He Q, Zeng Y, Cheng L.. Effectiveness of combined exercise in people with type 2 diabetes and concurrent overweight/obesity: a systematic review and meta-analysis. BMJ Open 2021;11:e046252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qadir R, Sculthorpe NF, Todd T, Brown EC.. Effectiveness of resistance training and associated program characteristics in patients at risk for type 2 diabetes: a systematic review and meta-analysis. Sports Med Open 2021;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jarvie JL, Pandey A, Ayers CR, et al. Aerobic fitness and adherence to guideline-recommended minimum physical activity among ambulatory patients with type 2 diabetes mellitus. Diabetes Care 2019;42:1333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao G, Ford ES, Li C, Balluz LS.. Physical activity in U.S. older adults with diabetes mellitus: prevalence and correlates of meeting physical activity recommendations. J Am Geriatr Soc 2011;59:132–7. [DOI] [PubMed] [Google Scholar]
- 9. Wang Y, Nie J, Ferrari G, et al. Association of physical activity intensity with mortality: a national cohort study of 403 681 US adults. JAMA Intern Med 2021;181:203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lear SA, Hu W, Rangarajan S, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet 2017;390:2643–54. [DOI] [PubMed] [Google Scholar]
- 11. Ding D, Kolbe-Alexander T, Nguyen B, et al. The economic burden of physical inactivity: a systematic review and critical appraisal. Br J Sports Med 2017;51:1392–409. [DOI] [PubMed] [Google Scholar]
- 12. Ding D, Lawson KD, Kolbe-Alexander T, et al. ; Lancet Physical Activity Series 2 Executive Committee. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet 2016;388:1311–24. [DOI] [PubMed] [Google Scholar]
- 13. Abu-Omar K, Rütten A, Burlacu I, et al. The cost-effectiveness of physical activity interventions: a systematic review of reviews. Prev Med Rep 2017;8:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li R, Zhang P, Barker LE, et al. Cost-effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care 2010;33:1872–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhong Y, Lin PJ, Cohen JT, et al. Cost-utility analyses in diabetes: a systematic review and implications from real-world evidence. Value Health 2015;18:308–14. [DOI] [PubMed] [Google Scholar]
- 16. Wu S, Cohen D, Shi Y, et al. Economic analysis of physical activity interventions. Am J Prev Med 2011;40:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021;372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention. Economic Evaluation Overview. 2021. Available at: https://www.cdc.gov/policy/polaris/economics/index.html (accessed 3 January 2022).
- 19. Marseille E, Larson B, Kazi DS, et al. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ 2015;93:118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Husereau D, Drummond M, Petrou S, et al. ; CHEERS Task Force. Consolidated health economic evaluation reporting standards (CHEERS) statement. Value Health 2013;16:e1–5. [DOI] [PubMed] [Google Scholar]
- 21. The World Bank. PPP conversion factor, GDP (LCU per international $). 2022. Available at: https://data.worldbank.org/indicator/PA.NUS.PPP (accessed 10 February 2022).
- 22. Shields GE, Elvidge J.. Challenges in synthesising cost-effectiveness estimates. Syst Rev 2020;9:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaplan R, Atkins C, Wilson D.. The cost-utility of diet and exercise interventions in non-insulin-dependent diabetes mellitus. Health Promot Int 1987;2:331–40. [DOI] [PubMed] [Google Scholar]
- 24. Kuo S, Ye W, de Groot M, et al. Cost-effectiveness of community-based depression interventions for rural and urban adults with type 2 diabetes: projections from program ACTIVE (adults coming together to increase vital exercise) II. Diabetes Care 2021;44:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pepin MJ, Valencia WM, Bettger JP, et al. Impact of supervised exercise on one-year medication use in older veterans with multiple morbidities. Gerontol Geriatr Med 2020;6:2333721420956751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coyle D, Coyle K, Kenny GP, et al. Cost-effectiveness of exercise programs in type 2 diabetes. Int J Technol Assess Health Care 2012;28:228–34. [DOI] [PubMed] [Google Scholar]
- 27. Johnson ST, Lier DA, Soprovich A, et al. How much will we pay to increase steps per day? Examining the cost-effectiveness of a pedometer-based lifestyle program in primary care. Prev Med Rep 2015;2:645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brun J-F, Bordenave S, Mercier J, et al. Cost-sparing effect of twice-weekly targeted endurance training in type 2 diabetics: a one-year controlled randomized trial. Diabetes Metab 2008;34:258–65. [DOI] [PubMed] [Google Scholar]
- 29. Marios T, Smart NA, Dalton S.. The effect of tele-monitoring on exercise training adherence, functional capacity, quality of life and glycemic control in patients with type II diabetes. J Sports Sci Med 2012;11:51–6. [PMC free article] [PubMed] [Google Scholar]
- 30. Sultana F, Saha S, Lsmail MS, et al. S. Cost-effective exercise programs on health-status of Malaysian diabetic individuals: a socio-psychological analysis. Int J Life Sci Pharma Res 2018;214–22. [Google Scholar]
- 31. Taylor AH, Taylor RS, Ingram WM, et al. Adding web-based behavioural support to exercise referral schemes for inactive adults with chronic health conditions: the e-coachER RCT. Health Technol Assess 2020;24:1–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Di Loreto C, Fanelli C, Lucidi P, et al. Make your diabetic patients walk: long-term impact of different amounts of physical activity on type 2. Diabetes Care 2005;28:1295–302. [DOI] [PubMed] [Google Scholar]
- 33. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dale LP, Vanderloo L, Moore S, Faulkner G.. Physical activity and depression, anxiety, and self-esteem in children and youth: an umbrella systematic review. Mental Health Phys Act 2019;16:66–79. [Google Scholar]
- 35. Rosenbaum S, Tiedemann A, Sherrington C, et al. Physical activity interventions for people with mental illness: a systematic review and meta-analysis. J Clin Psychiatry 2014;75:964–74. [DOI] [PubMed] [Google Scholar]
- 36. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016;316:1093–103. [DOI] [PubMed] [Google Scholar]
- 37. Li R, Qu S, Zhang P, et al. Economic evaluation of combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the community preventive services task force. Ann Intern Med 2015;163:452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.